��Ŀ����
��ͼ��ʵ���ҳ��õ�ʵ��������װ�ã�������ѧ֪ʶ�ش��������⣺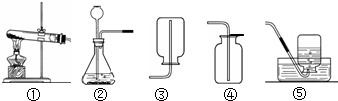
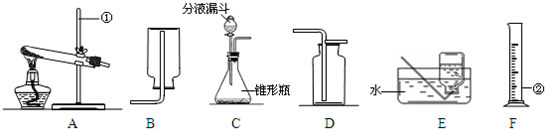
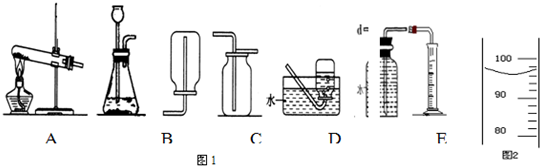
��1��������ͼ��ʾװ�ã�����������������ʵ������ȡ�������
A���âڢ���ȡ����B���âڢ���ȡ������̼
C���â٢���ȡ����������������D���âڢ���ȡ������̼
��2������Aװ�������������䷴Ӧ�Ļ�ѧ����ʽΪ
��3��װ�â�Ҳ������������������ƿ�еĹ����������
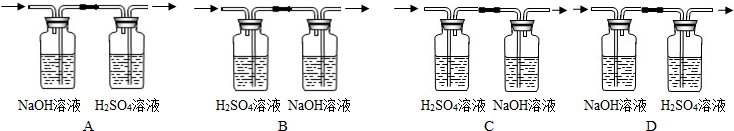
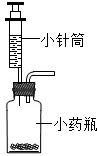
��4��С��ͬѧ��λ���ڶ��Խ��ѧ��������װ�âڽ���һϵ�иĽ��봴�£���ͼA��B��
Bװ�õ��ŵ���
Cװ�õ��ŵ���
��Aװ����ʾ�Ļ�ѧ��Ӧ��״̬Ϊ
��������С�����ֹͣ������
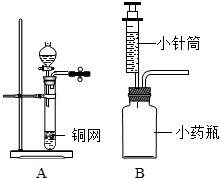
����Aװ����ȡ������̼��ͭ���Ϸŵ�ҩƷӦΪ
���������ݳ�������ķ���װ�ú��ռ�װ�õ�ѡ�����ݡ�ҩƷ��ԭ������ע��������з������
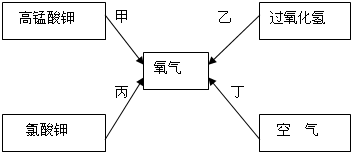
����⣺��1���â٢���Ͽ��ø��������ȡ�������âڢܸ��Ͽ���ʯ��ʯ��ϡ������ȡ������̼��
��2�����������ȡ�����Ļ�ѧ����ʽΪ��2KMnO4
K2MnO4+MnO2+O2���Թܿڷ�һ������Ŀ���Ƿ�ֹ������ط�ĩ���뵼�ܣ�
��3��װ�âڿ�����˫��ˮ�Ͷ�������������������ƿ�еĹ����ж������̣�������Ϊ�ӿ�˫��ˮ�ķֽ��ٶȣ�������ã���ѧ����ʽΪ2H2O2
2H2O+O2���������л���HCl��ˮ������Ҫ��ȥ����Ӧ��ͨ������������Һ��ȥ
HCl��Ȼ��ͨ��Ũ��������ˮ�֣�ͬʱ������Ҫ�쵽Һ�����£�
��4���Ա�����װ�õIJ�ͬ��ɿ���Bװ�õ��ŵ��ǿ���ʹ��Ӧ��ʱ��������ʱֹͣ��Cװ�õ��ŵ��� �������ã���Լ��Դ�����ɿ��Ʒ�Ӧ���ʣ�װ��A��Һ�嵹��������©���У���Һ���룬���Ա�ʾ��Ӧ��ֹͣ����Aװ����ȡ������̼��ͭ���Ϸŵ�ҩƷӦ�ǹ������ʯ����Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+CO2��+H2O��ͭ��������������Ϊ���������ǰ�������ᷴӦ�����������ʲ����ĺ����ʹ�ƵõĶ�����̼�л���������
�ʴ�Ϊ����1��CD��
��2��2KMnO4
K2MnO4+MnO2+O2����ֹ������ط�ĩ���뵼��
��3�������ã�2H2O2
2H2O+O2����D
��4������ʹ��Ӧ��ʱ��������ʱֹͣ���������ã���Լ��Դ�����ɿ��Ʒ�Ӧ����
��ֹͣ��ʯ��ʯ�������ʯ����CaCO3+2HCl=CaCl2+CO2��+H2O
ʹ�ƵõĶ�����̼�л������������ƵõĶ�����̼��������
��2�����������ȡ�����Ļ�ѧ����ʽΪ��2KMnO4
| ||
��3��װ�âڿ�����˫��ˮ�Ͷ�������������������ƿ�еĹ����ж������̣�������Ϊ�ӿ�˫��ˮ�ķֽ��ٶȣ�������ã���ѧ����ʽΪ2H2O2
| ||
HCl��Ȼ��ͨ��Ũ��������ˮ�֣�ͬʱ������Ҫ�쵽Һ�����£�
��4���Ա�����װ�õIJ�ͬ��ɿ���Bװ�õ��ŵ��ǿ���ʹ��Ӧ��ʱ��������ʱֹͣ��Cװ�õ��ŵ��� �������ã���Լ��Դ�����ɿ��Ʒ�Ӧ���ʣ�װ��A��Һ�嵹��������©���У���Һ���룬���Ա�ʾ��Ӧ��ֹͣ����Aװ����ȡ������̼��ͭ���Ϸŵ�ҩƷӦ�ǹ������ʯ����Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+CO2��+H2O��ͭ��������������Ϊ���������ǰ�������ᷴӦ�����������ʲ����ĺ����ʹ�ƵõĶ�����̼�л���������
�ʴ�Ϊ����1��CD��
��2��2KMnO4
| ||
��3�������ã�2H2O2
| ||
��4������ʹ��Ӧ��ʱ��������ʱֹͣ���������ã���Լ��Դ�����ɿ��Ʒ�Ӧ����
��ֹͣ��ʯ��ʯ�������ʯ����CaCO3+2HCl=CaCl2+CO2��+H2O
ʹ�ƵõĶ�����̼�л������������ƵõĶ�����̼��������
�����������漰����������������������̼��������ʵ��װ�á���ȡ��ԭ������ȡ���ռ�װ�õ�ѡ���Լ�������ӡ�װ����ȱ��Աȵ�֪ʶ�㣮Ӧ��ȷ��ǵ��͵������ʵ������ȡԭ���ͷ�Ӧ�Ļ�ѧ����ʽ��д���й�֪ʶ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
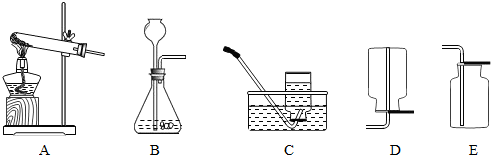
 ��3��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ