��Ŀ����
��������������Ϣ��д������Ҫ��Ļ�ѧ����ʽ����1���ڻ�ҩ���ҹ��Ŵ��Ĵ���֮һ��������ľ̿����ۡ�������KNO3����һ��������϶��ɣ���ը�������ء������Ͷ�����̼��
��2����ɫֲ��Ľո�[��Ҫ�ɷ�����ά�أ��仯ѧʽΪ[��C6H10O5��n]�������ʵ������������£�����ˮ��Ӧ���������ǣ���ѧʽΪC6H12O6���������������ʵ������������£���ת��Ϊ�Ҵ���C2H5OH���Ͷ�����̼����д������ά�����Ҵ��Ļ�ѧ��Ӧ����ʽ��
���𰸡���������д��ѧ����ʽʱ��������Ϥ��Ӧ�������ͷ�Ӧ�������������������غ㶨�ɡ����ݿ���ʵ��
����⣺��1��̼�����������ڱ�ը�����·�Ӧ�����ء������Ͷ�����̼��
�÷�Ӧ�Ļ�ѧ����ʽΪ��3C+S+2KNO3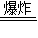 K2S+3CO2��+N2����
K2S+3CO2��+N2����
��2����ά�غ�ˮ�ڴ����������·�Ӧ���������ǣ�
�÷�Ӧ�Ļ�ѧ����ʽΪ����C6H10O5��n+nH2O nC6H12O6��
nC6H12O6��
�������ڴ����������·�Ӧ�����Ҵ��Ͷ�����̼��
�÷�Ӧ�Ļ�ѧ����ʽΪ��C6H12O6 2C2H5OH+2CO2����
2C2H5OH+2CO2����
�ʴ�Ϊ��
��1��3C+S+2KNO3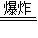 K2S+3CO2��+N2����
K2S+3CO2��+N2����
��2����C6H10O5��n+nH2O nC6H12O6��C6H12O6
nC6H12O6��C6H12O6 2C2H5OH+2CO2����
2C2H5OH+2CO2����
������������Ҫ���黯ѧ����ʽ����д���ѶȽϴ�
����⣺��1��̼�����������ڱ�ը�����·�Ӧ�����ء������Ͷ�����̼��
�÷�Ӧ�Ļ�ѧ����ʽΪ��3C+S+2KNO3
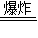 K2S+3CO2��+N2����
K2S+3CO2��+N2������2����ά�غ�ˮ�ڴ����������·�Ӧ���������ǣ�
�÷�Ӧ�Ļ�ѧ����ʽΪ����C6H10O5��n+nH2O
 nC6H12O6��
nC6H12O6���������ڴ����������·�Ӧ�����Ҵ��Ͷ�����̼��
�÷�Ӧ�Ļ�ѧ����ʽΪ��C6H12O6
 2C2H5OH+2CO2����
2C2H5OH+2CO2�����ʴ�Ϊ��
��1��3C+S+2KNO3
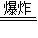 K2S+3CO2��+N2����
K2S+3CO2��+N2������2����C6H10O5��n+nH2O
 nC6H12O6��C6H12O6
nC6H12O6��C6H12O6 2C2H5OH+2CO2����
2C2H5OH+2CO2����������������Ҫ���黯ѧ����ʽ����д���ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ