��Ŀ����
��1����5�֣�����������һЩ�������漰�������ռ����������������ʯ�ҡ�����Ŷ����ݰ�ˮ�ȣ��������пո���������Ӧ����š�
1�������������_____________�� 2���������������_________��
3����Ϊ�к�θ��ҩ��_________�� 4������ë֯���ϴ�Ӽ�_______��
5�������к���������Ϊ���к���һ�ֶ������к��ļ�____________��
��2����5�֣�A��B��C����Ԫ�ص����ӽṹʾ��ͼ����ͼ��ʾ

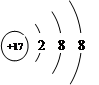
A B C
��A��BΪԭ��ʱ��X= ��Y= ��C��ʾ�����ӷ����� ����Ԫ����BԪ���γɵĻ��������� ������ӡ�����ԭ�ӡ��������ӡ������ɵģ��仯ѧʽΪ______ ___��
��1����5�֣��٣�2���ۣ�3���ڣ�4���ݣ�5����
(2)��5�֣�8 1 Cl- ���� NaCl
����

��ϰ��ϵ�д�
�����Ŀ



 �ӡ����������ɵģ��仯ѧʽΪ______ ___��
�ӡ����������ɵģ��仯ѧʽΪ______ ___��