��Ŀ����
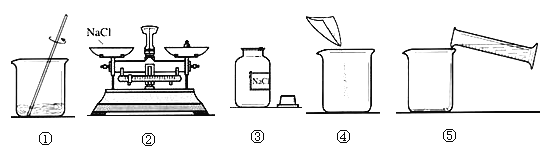
����Ŀ����9�֣��ڡ����뻯ѧ����У�ij��ȤС���ͬѧ��ʵ�����ú���������ʯ�ҵ�NaCl��������һ������������NaCl��Һ��ʵ�鲽������ͼ��ʾ��
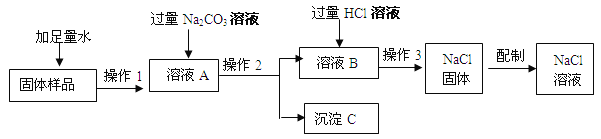
��ش�
��1���ڡ�������Ʒ���м���ˮʱ��������Ӧ�Ļ�ѧ����ʽΪ ��
��2������ҺA������������ ���ѧʽ�������롰����Na2CO3����������Ӧ�Ļ�ѧ����ʽ ��
��3���ڡ�����2���У�Ҫ�õ��IJ������������������ձ��⣬���� ��
��4�����롰����HCl��Һ����Ŀ���� ��
��5��������3���������� ��
��6����Ҫ����100g��������Ϊ10%���Ȼ�����Һ����Ҫˮ������ g����Ҫ�õ����������⣬����Ҫ�IJ��������� ��
���𰸡���1�� CaO + H2O== Ca��OH��2 ��1�֣� ����2��Ca��OH��2 NaCl ��2�֣����һ��1�֣�
Ca��OH��2 + Na2CO3== CaCO3�� + 2NaOH ��1�֣�����3��©����1�֣���
��4����ȥNa2CO3��NaOH���ʣ�1�֣�����5������ ��1�֣���
��6��90��1�֣�����Ͳ�ͽ�ͷ�ιܣ�ȫ�ԲŸ�1�֣���
��������
�����������1����ʯ�Ҿ���CaO����������ˮ��Ӧ����2��CaO��ˮ��Ӧ�IJ�����Ca��OH��2��������ҺA����Ca��OH��2��NaCl��Ca��OH��2��Na2CO3���ϸ��ֽⷴӦ�������������������ᷢ����Ӧ����3������2�ǹ��˲��������ݹ��˲�����Ҫ�õ��������������ɣ���4����Ϊ��ҺA�����ӵ�Na2CO3��Һ�ǹ����ģ�������ҺB����ʣ���Na2CO3�����ɵ�NaOH������������������Ϊ�˳�ȥNa2CO3��NaOH��ͬʱ�������µ����ʣ���5����ҺB�м����������ᣬ�õ�����NaCl��Һ��Ϊ�˵õ�NaCl���壬��Ҫ������������6����Ҫˮ������Ϊ��100g��10%=10g����ˮ������Ϊ��100g-10g=90g����������һ������������NaCl��Һ����Ҫ�������������ɣ���Ҫ�������У�������ƽ���ձ�����Ͳ������������ͷ�ι���

����Ŀ����ѧ��ȤС���ڻ�ѧʵ���ҷ�����һƿ�Ѳ��ֱ��ʵ�����������Һ����������ı��������С��ͬѧȡ10g����Һ�����ձ��У�Ȼ��20gϡ�����4�μ���ͬһ���ձ��г�ַ�Ӧ����Ӧ�����������
���ʵ� ���Ĵ� ÿ�η�Ӧ��
NaOH��Һ ����ϡ���� ��������
ʵ����̵����ݼ�¼���£�
ʵ����� | 1 | 2 | 3 | 4 |
ϡ�����������g�� | 5 | 5 | 5 | 5 |
��Ӧ���ձ������ʵ����� | 15 | 19.56 | 24.12 | 29.12 |
����һ��ʵ��ǡ����ȫ��Ӧ����ش��������⣺
��1��д�����Ĵ�ʵ�����Һ�����ʵĻ�ѧʽ____________��
��2��д���ڶ���ʵ���з�Ӧ�Ļ�ѧ����ʽ___________________________________��
��3��������֪�������г����μӷ�Ӧ��̼����������x���ı���ʽ_____________��
��4������ϡ�����������������Ϊ___________��
��5��������������Һ�б��ʵ�����������ʣ���������Ƶ�������Ϊ______________��
��6�������η�Ӧ�����Һ�У��ټ���5.88gˮ��������Һ�����ʵ���������Ϊ_____��
����Ŀ���±���һЩʳ���pH��θ�����IJ��˿ո�ʱ����ʳ�õ��ǣ� ��
���� | ���� | ���� | ţ�� | ������ |
pH | 3��4 | 3.5��4.5 | 6.3��6.6 | 6.8��8.0 |
A������ B������ C��ţ�� D��������