��Ŀ����
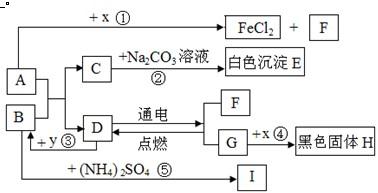
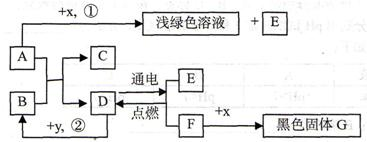
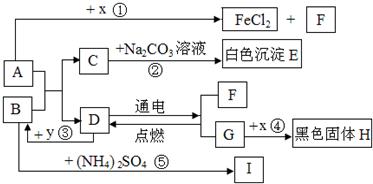
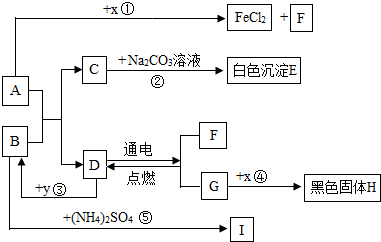
A��B�ɷ����кͷ�Ӧ��D�������������Һ�壮ͨ������£�F��G��IΪ���壬��F��һ�������Դ��x��ĿǰӦ����㷺�Ľ�����y������ʳƷ������������ʼ��ת����ϵ��ͼ��ʾ�����������ȥ����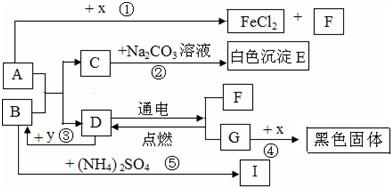
��1��C�Ļ�ѧʽ��
��2��B�׳�
��3����Ӧ�۷�Ӧʱ�ų��������ȣ���ѧ����ʽΪ
��Ӧ�ܻ�ѧ����ʽΪ
��4����ѧ���о����ʼ���仯�Ŀ�ѧ���ȽϷ�Ӧ�ۡ��ܣ���Ի�ѧ�仯����ʶ�ǣ���д2����
�����������������D�������������Һ�壬����ȷ��DΪˮ��Ȼ�����������Ĺ�ϵͼ���ó��������ʣ������ƶ���ʱ��һ��Ҫע�������֮�����ϵ��
����⣺��D�������������Һ�壬�ƶ�DΪˮ������F��G��F��������G������������y������ʳƷ��������ܸ�ˮ��Ӧ���ų��������������ǿ���ȷ��YΪ�����ƣ���BΪ�������ƣ���IΪ������x��ĿǰӦ����㷺�Ľ���������XΪ��������A������Ӧ�����Ȼ�����������������AΪ���ᣮ��CΪ�Ȼ��ƣ�EΪ̼��ƣ�
�ʴ�Ϊ��
��1��CaCl2��H2
��2����ʯ�ң�����ʯ�ң�������
��3��CaO+H2O�TCa��OH��2��3Fe+2O2
Fe3O4
��4�������������ɣ��������仯�����������仯��
�ʴ�Ϊ��
��1��CaCl2��H2
��2����ʯ�ң�����ʯ�ң�������
��3��CaO+H2O�TCa��OH��2��3Fe+2O2
| ��ȼ |
| �T |
��4�������������ɣ��������仯�����������仯��
��������ѧ�仯������һ�������µ����ʣ������ڻ�ѧ�仯�Ĺ��������������������ı仯������Ҫ��ѧ������������Щ�仯�Ƿ��ȵģ���Щ�仯�����ȣ�

��ϰ��ϵ�д�
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
�����Ŀ