��Ŀ����
������з�Ӧ�Ļ�ѧ����ʽ��
(1)ʵ�����ü��Ȱ���ɫ����������____________________________
(2)ľ̿�����»�ԭ����ͭ_______________________________
(3)��ʯ��ˮ���������̼_______________________________
(4)ú�ڿ�������ȫȼ��______________________________
(5)��ˮ���ն�����̼ʱ��������Ӧ�Ļ�ѧ����ʽΪ_____________
(6)DZˮͧ�����ù������ƣ���ѧʽNa2O2���Ͷ�����̼��Ӧ����̼���ƺ�һ�ֿɹ�����������_________________________
(7) ��֪��ȼ��þ�����ڴ����Ķ�����̼�м���ȼ�գ�����һ�ְ�ɫ��ĩ�ͺ�ɫ���ʣ�д����Ӧ�Ļ�ѧ����ʽ____________________
(1)ʵ�����ü��Ȱ���ɫ����������____________________________
(2)ľ̿�����»�ԭ����ͭ_______________________________
(3)��ʯ��ˮ���������̼_______________________________
(4)ú�ڿ�������ȫȼ��______________________________
(5)��ˮ���ն�����̼ʱ��������Ӧ�Ļ�ѧ����ʽΪ_____________
(6)DZˮͧ�����ù������ƣ���ѧʽNa2O2���Ͷ�����̼��Ӧ����̼���ƺ�һ�ֿɹ�����������_________________________
(7) ��֪��ȼ��þ�����ڴ����Ķ�����̼�м���ȼ�գ�����һ�ְ�ɫ��ĩ�ͺ�ɫ���ʣ�д����Ӧ�Ļ�ѧ����ʽ____________________
(1) 2KMnO4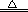 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��
(2) C+ 2CuO 2Cu + CO2��
2Cu + CO2��
(3)Ca(OH)2+CO2===CaCO3��+H2O
(4) C + O2 CO2
CO2
(5) H2O + CO2="=" H2CO3
(6) 2Na2O2+2CO2==2Na2CO3+O2
(7) 2Mg+CO2 2MgO+C
2MgO+C
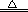 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2�� (2) C+ 2CuO
 2Cu + CO2��
2Cu + CO2�� (3)Ca(OH)2+CO2===CaCO3��+H2O
(4) C + O2
 CO2
CO2(5) H2O + CO2="=" H2CO3
(6) 2Na2O2+2CO2==2Na2CO3+O2
(7) 2Mg+CO2
 2MgO+C
2MgO+C���⿼�������д��ѧ����ʽ����ѧ����ʽ����дԭ��Ҫ�Կ���ʵΪ���ݣ�����ƾ��������ʵ�ϲ����ڵ����ʺͻ�ѧ��Ӧ���ڱ������������غ㶨�ɣ�Ҫʹ��Ӧǰ���ԭ�ӵ���������Ŀ���ֲ��䡣
(1)ʵ�����ü��Ȱ���ɫ����������������������ڼ��������·�Ӧ��������ء��������̺��������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ2KMnO4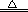 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
(2)ľ̿�����»�ԭ����ͭ����ͭ�Ͷ�����̼���ʸ÷�Ӧ�Ļ�ѧ����ʽΪC+ 2CuO 2Cu + CO2����
2Cu + CO2����
(3)������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ���ʸ÷�Ӧ�Ļ�ѧ����ʽΪCa(OH)2+CO2=CaCO3��+H2O��
(4)ú�ڿ�������ȫȼ�����ɶ�����̼���ʸ÷�Ӧ�Ļ�ѧ����ʽΪC + O2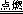 CO2��
CO2��
(5)��ˮ���ն�����̼ʱ����������̼��ˮ��Ӧ����̼�ᣬ�ʷ�����Ӧ�Ļ�ѧ����ʽΪH2O + CO2=H2CO3��
(6)DZˮͧ�����ù������ƣ���ѧʽNa2O2���Ͷ�����̼��Ӧ����̼���ƺ�һ�ֿɹ������������������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��
(7) ��֪��ȼ��þ�����ڴ����Ķ�����̼�м���ȼ�գ�����һ�ְ�ɫ��ĩ����þ�ͺ�ɫ����̼���ʸ÷�Ӧ�Ļ�ѧ����ʽΪ2Mg+CO2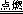 2MgO+C��
2MgO+C��
(1)ʵ�����ü��Ȱ���ɫ����������������������ڼ��������·�Ӧ��������ء��������̺��������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ2KMnO4
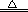 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����(2)ľ̿�����»�ԭ����ͭ����ͭ�Ͷ�����̼���ʸ÷�Ӧ�Ļ�ѧ����ʽΪC+ 2CuO
 2Cu + CO2����
2Cu + CO2����(3)������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ���ʸ÷�Ӧ�Ļ�ѧ����ʽΪCa(OH)2+CO2=CaCO3��+H2O��
(4)ú�ڿ�������ȫȼ�����ɶ�����̼���ʸ÷�Ӧ�Ļ�ѧ����ʽΪC + O2
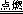 CO2��
CO2��(5)��ˮ���ն�����̼ʱ����������̼��ˮ��Ӧ����̼�ᣬ�ʷ�����Ӧ�Ļ�ѧ����ʽΪH2O + CO2=H2CO3��
(6)DZˮͧ�����ù������ƣ���ѧʽNa2O2���Ͷ�����̼��Ӧ����̼���ƺ�һ�ֿɹ������������������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��
(7) ��֪��ȼ��þ�����ڴ����Ķ�����̼�м���ȼ�գ�����һ�ְ�ɫ��ĩ����þ�ͺ�ɫ����̼���ʸ÷�Ӧ�Ļ�ѧ����ʽΪ2Mg+CO2
 2MgO+C��
2MgO+C��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
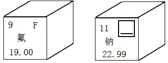 ��1����ͼ������Ԫ����Ԫ�����ڱ��е���Ϣ�����з����
��1����ͼ������Ԫ����Ԫ�����ڱ��е���Ϣ�����з����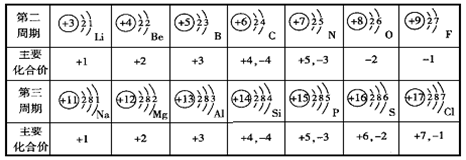
 ���ϱ��и�Ԫ�ص���������ϼ���ԭ�������������Ĺ�ϵ�� �����ȡ�����ȡ�����
���ϱ��и�Ԫ�ص���������ϼ���ԭ�������������Ĺ�ϵ�� �����ȡ�����ȡ�����