��Ŀ����
��10����20����(4��)��ش������йؽ��������⡣(1)���ǻ��ý�����Ϊʲôͨ������ȴ����ʴ?
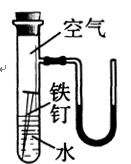
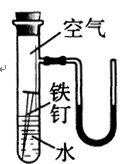
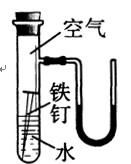
(2)�ұ���ij̽��ʵ��װ��ͼ��һ��ʱ����ܹ۲쵽ʲô����?
(װ�����������ã��ҿ�ʼʱU�����˵ĺ�īˮҺ����ƽ)
(3)X��Y��Z�����ֽ������壬��X��Y����ϡ�����У�Y�ܽⲢ����������X�ޱ仯����X��Z������������Һ�У�X��������������Z�ޱ仯��
���ж�X��Y��Z�������ֽ����Ļ����ǿ������˳��
�ھ���ȷ��һ��X��д��X����������Һ��Ӧ�Ļ�ѧ����ʽ��
(4��)(1)����������Ӧ��������������ܵ���������Ĥ�𱣻����á�
(2)�������⣬U��Һ���Ϊ����ҵ͡�
(3)��Y��x��Ag��z ��Cu+2AgN03=Cu(N03)2+2Ag����:
��
(2)�������⣬U��Һ���Ϊ����ҵ͡�
(3)��Y��x��Ag��z ��Cu+2AgN03=Cu(N03)2+2Ag����:
��

��ϰ��ϵ�д�
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
�����Ŀ