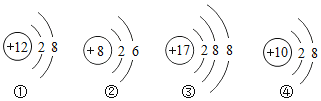
��Ŀ����
������ͼ�ش����⡣
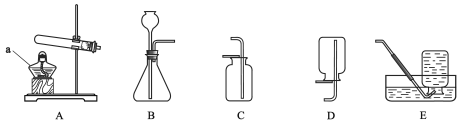
(1)����a��������_____��
(2)ʵ�����ø��������ȡ�����ķ��ű���ʽ��_____���÷�Ӧ�Ļ�������Ϊ����ѡ�õķ���װ����_____(����ţ���ͬ)�����ռ�װ����_____��E��
(3)ʵ���һ����ù��������Ʊ���������ѡ�õķ���װ��Ϊ____��
(4)���ȸ����������ˮ���ռ�ʱ����______________ʱ����ʼ�ռ���������ƿ�е�ˮ�������___�ò���Ƭ��סƿ�ڣ�С�ĵذ�ƿ���Ƴ�ˮ�ۣ�_______�������ϡ�
(5)����ˮ���ռ������漰���¹ؼ����裺�ټ�������ԣ���װҩ�̶������ռ����ܼ��ȣ���Ϩ��ƾ��ƣ���ˮ���г��������ܡ���ȷ��˳����____(����ĸ���)��
A.�ڢ٢ۢܢݢ� B. �ۢܢڢ٢ޢ� C. �٢ڢܢۢޢ� D. �٢ۢڢܢݢ�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д� ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д���1��С���ȼ�λͬѧ�����м������ʯ��ˮ������������Һ��ʵ��̽����������룺
��������⣩��μ�����������ɫ��Һ��
��ʵ�鷽�������ǽ�������ͼ��ʾ��ʵ��
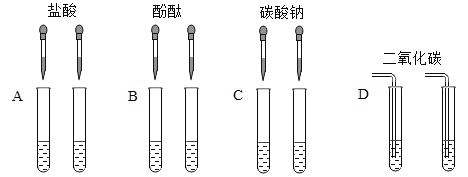
����ش��������⣺
������ʵ���в��ܴﵽʵ��Ŀ����___________������ĸ����
��C��ʵ���з�Ӧ�Ļ�ѧ����ʽΪ________________________��
��D��ʵ���б���ǵ�ԭ��Һ��_______________��
������̽����ʵ�������С��ͬѧ��A��B��C��D�����Թ��е�����ȫ������ͬһ���ɾ����ձ��У���ַ�Ӧ�õ���ɫ����������Һ���Ը���Һ�ijɷ��ֽ�����̽����
��������⣩����Һ�г�ˮ����̪�������Щ���ʣ�
���������ϣ��Ȼ�����Һ�����ԣ�
����������裩
��_______________
��NaCl��CaCl2��HCl
��NaCl��CaCl2��NaOH
����˼����չ�������������������ֻ��һ��������������_____������ţ���������________��
�ڸ�����ѧ��ѧ֪ʶ����֤�ձ�����Һ�п����е������Ƿ���ڣ�������Щ���ʵ���ʹ�ò�����ɸ�ʵ��___________������ĸ��
a pH��ֽ b ��������Һ c ��ɫʯ����Һ d ͭ e ��������������Һ��
��2����������������������������Ҫ��Դ��
��������⣩�����ǵ�ȼ�ղ�����CO2��H2O���ɴ��ܷ�֤����������ֻ��̼Ԫ�غ���Ԫ����ɵ��л��
�����ʵ�飩Ϊ��ȷ�������ǵ�Ԫ����ɣ�ijС�����������ʵ�飨����Ũ���ᡢ��ˮCaCl2��Ϊ���ø���������̶ֹ�װ��ʡ�ԣ���
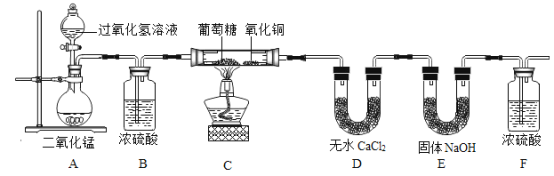
��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��__________________________��
��װ��B��Ũ�����������_________________________��
��װ��C������ͭ��������_________________________��
���������ۣ�
���ó��п����Ĵ��������װ��A���Ƿ��������ʵ��Ľ��У�_____����ǡ���ԭ����______________________��
��װ��C��������ȼ�յ�����ص���_________________������һ������
�����ݴ������±���ͬѧ����д��ʵ�鱨�棬���������ɡ�
ʵ����ʵ | ���ݷ��������� |
1.8g��������ȫȼ�գ��õ�2.64gCO2��1.08gH2O | ����___________________________ ���ۣ������Ǻ���C��H��O����Ԫ�� |
�����۽�����Ϊ�˾���������
��1����ʵ���ڽ��й�����Ӧע���������_______________��1������
��2���Ӷ���ʵ��ĽǶȿ�����ʵ��ɽ�һ���Ľ������Ҫд��һ���Ľ������________��
����ݱ���һЩʳ��Ľ���pHֵ���ж�����˵����ȷ����
ʳ�� | ����֭ | ƻ��֭ | ţ�� | ������ |
pH | 3.5-4.5 | 2.9-3.3 | 6.3-6.6 | 7.6-8.0 |
A.θ������߶�ʳƻ������
B.ţ������ζ��������
C.����ʳ��,������������ǿ
D.����֭��ʹ��ɫʯ����Һ���


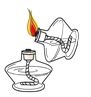 ��ȼ�ƾ��� B.
��ȼ�ƾ��� B. ��ĥ������ζ C.
��ĥ������ζ C.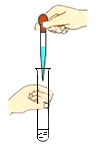 �μ�Һ�� D.
�μ�Һ�� D.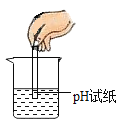 �ⶨ��Һ��pH
�ⶨ��Һ��pH