��Ŀ����
ȫ��۲���Ϊ�����������������������������ݡ��������ա���˾���죮��������¶�����ڼ���ɳĮ�Էɳɹ�������6.5��Ӣ�߸ߵ�ƽ������У�ʹ��Һ̬������ȼ�ϣ������ʱ��ɴ�һ�ܣ�ȫ��۲����п�������Ұ���ɹ۲�뾶600Ӣ��ķ�Χ��������۵ͣ���Ϊ��۸߰���������ǵ�����ߣ����������ա���˾�����ܲÿ����ڼ��ݰ��»��ȿվ����صĻ����ܷ�ʱ˵����������Ϊȼ�ϣ�����Դ�ܶ������͵�3�����������Ը��͵ijɱ��ɵø����ã����������ŷţ������˽⣬��ȫ��۲��ߡ�ֻ���ų�ˮ������
��1��д��һ����֪�����ܲ��������ķ������û�ѧ����ʽ��ʾ________��
��2���г��Ͼ�����������������Щ�����߳����Ѽ��������������������������Һ�����������õĸֹ��з�Ӧ������H2OҲ�Ƿ�Ӧ��֮һ��������NaAlO2����������д���÷�Ӧ�Ļ�ѧ����ʽ________��
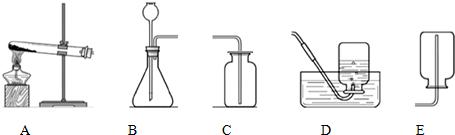
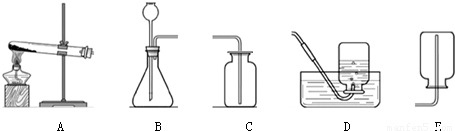
��3�������ʵ�����в������ַ�����ȡһ�����������������������У�ѡ����________������ţ���Ϊ���巢��װ�ã���________ ������ţ��ռ�������ԭ����________��Ҫ����ռ����������Ƿ����������õķ�����________��
��4�������ͼ��鶼����ɫ��ζ�Ŀ�ȼ���壬���Ը���________ �IJ�ͬ�������֣�
��5����֪��������Դ������Щ�����һ����________�����ձ�������º˵�վ��ը�����ϴ�ķ�������Ⱦ����Է�չ�˵��кο�����________��

�⣺��1��ʵ������������п��ϡ���ᣬ���߷�Ӧ��������п������������ʽΪ��Zn+H2SO4=ZnSO4 +H2����
��2��������Ϣ���Ѽ��������������������������Һ�����������õĸֹ��з�Ӧ������H2OҲ�Ƿ�Ӧ��֮һ��������NaAlO2��������֪����Ӧ���������������ơ�ˮ���֣�������ΪNaAlO2�����������Է���ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��3����Ϊ���壬����������ҺΪҺ�壬���ߵķ�Ӧ�����dz��£�����װ��ѡB�������������ܶȱȿ���С�����������ſ������ռ��������������ܽ���ˮ�������ռ���ˮ���������������ȵķ���Ϊ����С�Թ��ռ������ھƾ��ƻ����ϵ�ȼ���緢�����ı������������崿����
��4�������ͼ��鶼�п�ȼ�ԣ���������Ԫ����ɣ�ȼ�ղ�����ˮ��������̼��������Ԫ����ɣ�ȼ�ղ����Ƕ�����̼��ˮ���ʶ��߿�ͨ��ȼ�ղ������
��5�����ڿ���������Դ�ܶ࣬����ܡ�̫���ܡ������ܡ����ܵȣ����ں˵�վ��ը�����ϴ�ķ�������Ⱦ���ʷ�չ�˵�Ҫע���ֹй©��Ӧ��ǿ���У��������¼�����
�ʴ�Ϊ����1��Zn+H2SO4=ZnSO4 +H2����
��2��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��3��B��D��E��������������ˮ���������ܶȱȿ���С������С�Թ��ռ������ھƾ��ƻ����ϵ�ȼ���緢�����ı������������崿����
��4��ȼ�ղ��
��5��̫���ܣ���ǿ���У��������¼����ȣ�
��������1������ʵ������������ԭ���ش�
��2��������Ϣ���Ѽ��������������������������Һ�����������õĸֹ��з�Ӧ������H2OҲ�Ƿ�Ӧ��֮һ��������NaAlO2���������ش�
��3��������������������Һ��״̬�Ͷ��ߵķ�Ӧ�����ش��һ�գ����������ռ�װ��ѡ������ݺ����������ʻش�ڶ��գ������������ȵļ��鷽���ش��һ�գ�
��4�����������ͼ���ijɷֺͿ�ȼ�Ե����ʻش�
��5����������Դ�������ʹ�õ�ע������ش�
�������������ȡ�dz�����Ҫ�Ļ�ѧʵ��֮һ���������ص㣬���ǿ����ȵ㣬���ⷴӦԭ��������װ�õ�ѡ�����ݡ�����ļ��顢�������ռ��������˽�ʵ�鲽���ע�������ǽ����������Ĺؼ���
��2��������Ϣ���Ѽ��������������������������Һ�����������õĸֹ��з�Ӧ������H2OҲ�Ƿ�Ӧ��֮һ��������NaAlO2��������֪����Ӧ���������������ơ�ˮ���֣�������ΪNaAlO2�����������Է���ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��3����Ϊ���壬����������ҺΪҺ�壬���ߵķ�Ӧ�����dz��£�����װ��ѡB�������������ܶȱȿ���С�����������ſ������ռ��������������ܽ���ˮ�������ռ���ˮ���������������ȵķ���Ϊ����С�Թ��ռ������ھƾ��ƻ����ϵ�ȼ���緢�����ı������������崿����
��4�������ͼ��鶼�п�ȼ�ԣ���������Ԫ����ɣ�ȼ�ղ�����ˮ��������̼��������Ԫ����ɣ�ȼ�ղ����Ƕ�����̼��ˮ���ʶ��߿�ͨ��ȼ�ղ������
��5�����ڿ���������Դ�ܶ࣬����ܡ�̫���ܡ������ܡ����ܵȣ����ں˵�վ��ը�����ϴ�ķ�������Ⱦ���ʷ�չ�˵�Ҫע���ֹй©��Ӧ��ǿ���У��������¼�����
�ʴ�Ϊ����1��Zn+H2SO4=ZnSO4 +H2����
��2��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��3��B��D��E��������������ˮ���������ܶȱȿ���С������С�Թ��ռ������ھƾ��ƻ����ϵ�ȼ���緢�����ı������������崿����
��4��ȼ�ղ��
��5��̫���ܣ���ǿ���У��������¼����ȣ�
��������1������ʵ������������ԭ���ش�
��2��������Ϣ���Ѽ��������������������������Һ�����������õĸֹ��з�Ӧ������H2OҲ�Ƿ�Ӧ��֮һ��������NaAlO2���������ش�
��3��������������������Һ��״̬�Ͷ��ߵķ�Ӧ�����ش��һ�գ����������ռ�װ��ѡ������ݺ����������ʻش�ڶ��գ������������ȵļ��鷽���ش��һ�գ�
��4�����������ͼ���ijɷֺͿ�ȼ�Ե����ʻش�
��5����������Դ�������ʹ�õ�ע������ش�
�������������ȡ�dz�����Ҫ�Ļ�ѧʵ��֮һ���������ص㣬���ǿ����ȵ㣬���ⷴӦԭ��������װ�õ�ѡ�����ݡ�����ļ��顢�������ռ��������˽�ʵ�鲽���ע�������ǽ����������Ĺؼ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ