��Ŀ����
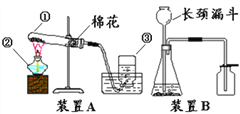
����Ŀ��ij��ѧ��ȤС��Ϊ�ⶨijʯ��ʯ��Ʒ(���ʲ�����ˮ��Ҳ�����ᷴӦ) ��̼��Ƶ�����������������ͼ��ʾ��ʵ�顣�����������Ϣ�ش���������:
(1) д����Ӧ�Ļ�ѧ����ʽ____________��
(2) �г�������Ʒ�вμӷ�Ӧ��̼�������(x) �ı���ʽ____________��
(3) ���ɵ��Ȼ��Ƶ�����Ϊ____________��
(4) ����Ʒ��̼��Ƶ���������Ϊ____________��
(5) ������ʯ��ʯ125t ��ȡ�����ʵ���ʯ�ҵ�����Ϊ____________��
���𰸡� CaCO3+2HCl=CaCl2+CO2��+H2O ![]() 22.2g 80% 56t
22.2g 80% 56t
��������(1)�����̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+CO2��+H2O��
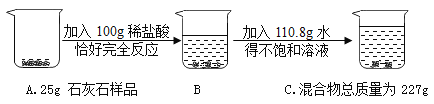
(2)������̼������Ϊ25g+100g+110.8g-227g=8.8g������Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
x 8.8g
![]()
x=20g��
��� ![]() ��
��
(3)�������Ȼ�������Ϊy��
CaCO3+2HCl=CaCl2+CO2��+H2O
111 44
y 8.8g
![]()
y=22.2g��
(4)����Ʒ��̼��Ƶ���������=![]() ��100%=80%��
��100%=80%��
(5)��������ʯ�ҵ�����Ϊz��
CaCO3![]() CaO+CO2��
CaO+CO2��
100 56
125t ��80% z
![]()
z=56t��
��������ʯ��ʯ125t ��ȡ�����ʵ���ʯ�ҵ�����Ϊ56t��

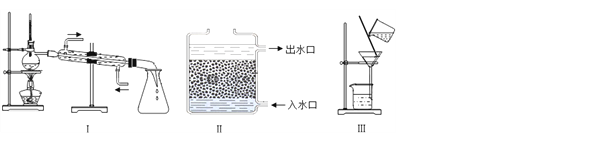
����Ŀ��(1)��ѧʵ�����������ǿ�ѧ��˼ά������Ҳ��ǿ������ѧϰ��ѧ����Ȥ��Ϊ�˱����о������ǿ��ѻ�ѧʵ�����ʵ��װ�á����ʵ����ʡ�̽���ķ������ȽǶȽ��з��ࡣ
��������ʵ��:
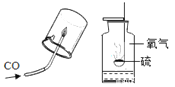
A�� | B�� | ʵ��C |
|
|
|
�����㽫ʵ��C ���࣬���ѡ���Ϊ_____��(����A������B��)��������____________��
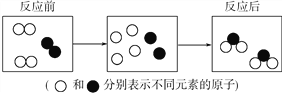
(2)��ѧ��Ӧ����������Ԫ�صĻ��ϼ۷����仯�ķ�Ӧ����������ԭ��Ӧ����H2+Cl2=2HCl����Ӧǰ����Ԫ�غ���Ԫ�صĻ��ϼ۷����˱仯���÷�Ӧ��������ԭ��Ӧ�������ж�Na2CO3+2HCl=H2O+CO2+2NaCl____(ѡ������������������)������ԭ��Ӧ��������ȷ��������Ϸ�Ӧ____________(ѡ����һ��������һ����������������)��������ԭ��Ӧ��