��Ŀ����
���� 4 �� 6 �գ�����������ij�������ĶԶ��ױ�װ�÷���©�ͱ�ը��������Ա���ˣ���ʧ���ء��Զ��ױ��Ļ�ѧʽ�� C8H10����ɫ��Һ�壬���з�����ζ����ȼ����������������γɱ�ը�Ի��� �����йضԶ��ױ���˵����ȷ���ǣ�������
A.�Զ��ױ�����������
B.�Զ��ױ�����Է��������� 106g
C.�Զ��ױ���̼��Ԫ��������Ϊ 4:5
D.һ���Զ��ױ��������� 8 ��̼ԭ�Ӻ� 10 ����ԭ�ӹ���
��ϰ��ϵ�д�
�����Ŀ
����������ʱ�Ӵ���������Ʒ��
��Ʒ | ������ | ����� |
��Ч�ɷ� | ��ԭ���� | ���� |
��1������������ʱ����һ��������������ԭ���������⣬�������ԭ����_____��
��2����ѡ�ý�������ˮ������Ҫ�ɷ���̼��ƣ����û�ѧ����ʽ��ʾ��ԭ��_____��


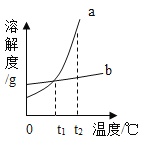
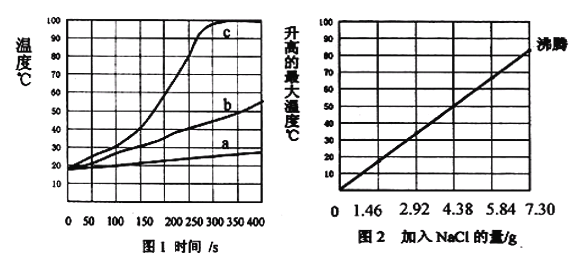
 ��ʱb�ı�����Һ��ˮ�ɱ�Ϊ��������Һ
��ʱb�ı�����Һ��ˮ�ɱ�Ϊ��������Һ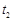 ��ʱa�ı�����Һ������
��ʱa�ı�����Һ������ b
b