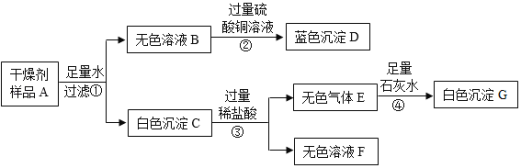
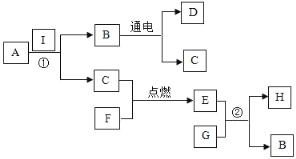
��Ŀ����
ʵ������һƿ����������Һ����ǩ��ͼ��

�Իش�:
��1��ʵ������ȡ����ʱ��ͨ��Ҫ��30%��H2O2��Һϡ�ͣ������ԭ����_______��
��2��ʵ��������6%�Ĺ���������Һ100g����30%��H2O2��Һ���Ϊ_____mL����ˮΪ_________mL ��
��3��ijͬѧȡ6.8g����ϡ�ͺ����Һ����KMnO4��Һ���ⶨ��Ũ�ȣ���Ӧ�Ļ�ѧ����ʽΪ2KMnO4+5H2O2+3H2SO4=K2SO4+2MnSO4+SO2��+8H2O��ǡ����ȫ��Ӧʱ��������KMnO4����Ϊ0.632g������ϡ�ͺ���Һ��ʵ��Ũ��_________��д��������̣���
��4������Һϡ��������ȷ�ģ���ʵ��Ũ��ƫ�͵Ŀ���ԭ����__________��
ij����������ʯ�ҳ�ȥ��Һ�в����ϡ���ᣬʹ��Һ�����Զ��ﵽ�ŷű���ijУ��ѧ��ȤС���ͬѧ��ȡ�˴�����ķ�Һ��Ʒ�����Ƕ����е����ʳɷֺܸ���Ȥ���������²��룺
����٣�ֻ��CaCl2����ڣ���CaCl2��Ca��OH��2
����ۣ���CaCl2��HCl����ܣ���CaCl2��HCl��Ca��OH��2
�������ϣ��Ȼ�����Һ�����ԡ�
̽�����̣�
ͬѧ�Ǿ�������ֱ�ӵó�����_____����ȷ��������_____��
С����Ϊ���������е�һ�����ʲ���Ҫ��֤��������_____��
Ϊ����֤���������Ƿ���ȷ��ͬѧ�Ƿ����ò�ͬ��������ʵ�飺
ʵ�鷽�� | ʵ������ | ʵ����� | |
���� | �ⶨ��Һ�����ȣ����������_____ | pH_____7������������������� | ����۳��� |
���� | ȡ����������ķ�Һ��Ʒ���Թ��У�����_____ | �Ȳ������ݣ���� �ְ�ɫ���� | ����۳��� |
���� | ȡ����������ķ�Һ��Ʒ���Թ��У������Ȼ�����Һ | _____ | ����ڲ����� |
��˼�����ۣ�
��1������Ϊ����ͬѧ�ܷ��������ʵ��ó�����۳�����������_____��
��2��Ϊ����֤����۳�����������ѡ����Լ���_____��
A ��ɫʯ����Һ B ��ɫ��̪��Һ C ��������Һ D ����
����ʵ������ͬѧ�Ƿ��ָó�������ķ�Һ��Ȼû�дﵽ�ŷű���
�ܽ���������
����Ϊ������Һ�в��������ʱ������ʹ�ù�����_____��


 �ⶨ������O2�ĺ���
�ⶨ������O2�ĺ��� ��֤��CO2��NaOH��Ӧ
��֤��CO2��NaOH��Ӧ �Ƚ�ͭ���������εĴ�Ч��
�Ƚ�ͭ���������εĴ�Ч�� ��װ�þ��С����շ��������Ĺ���
��װ�þ��С����շ��������Ĺ���