��Ŀ����
��2013?�Ͼ���ģ����1���Ͼ���ɽ����Ȫ�ȼٴ����������еĺ�ȥ����
����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����
A������ B��ԭ�� C��Ԫ��
�ڼ������Ȫˮ��Ӳˮ������ˮ�ļ�����
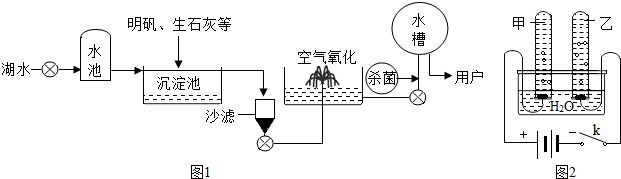
��2���Ͼ�ij������ˮ������������ͼ1��ʾ��
��������������ˮ����ʹ�õľ�ˮ������
A������ B������ C����� D������
���ڳ������У���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O�THClO+
��3��ˮ��������ܼ����������������ʷֱ����ˮ�У������γ���Һ����
A��ֲ���� B������ C������ D���������
��4��ˮ����Ȼ�����в��ֽ⣬����ͨ��������¿��Էֽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ
����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����
C
C
������ĸ����A������ B��ԭ�� C��Ԫ��
�ڼ������Ȫˮ��Ӳˮ������ˮ�ļ�����
ȡ�����������ˮ�������۲����������ĭ�϶࣬����ˮ�����û����ĭ����ĭ���٣���Ӳˮ
ȡ�����������ˮ�������۲����������ĭ�϶࣬����ˮ�����û����ĭ����ĭ���٣���Ӳˮ
����2���Ͼ�ij������ˮ������������ͼ1��ʾ��
��������������ˮ����ʹ�õľ�ˮ������
AB
AB
������ĸ����A������ B������ C����� D������
���ڳ������У���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ��
CaO+H2O=Ca��OH��2
CaO+H2O=Ca��OH��2
��������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������Cl2+H2O�THClO+
HCl
HCl
����3��ˮ��������ܼ����������������ʷֱ����ˮ�У������γ���Һ����
A
A
A��ֲ���� B������ C������ D���������
��4��ˮ����Ȼ�����в��ֽ⣬����ͨ��������¿��Էֽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ
2H2O
2H2��+O2��
| ||
2H2O
2H2��+O2��
����ͼ2��ʾ��װ���У�����Դ��ͨһ��ʱ����������������ҹ�����������֮��Ϊ
| ||
1��2
1��2
��
�������÷���ˮ����������ˮ��Ӳˮ��
����ˮ����ʹ�õľ�ˮ����ͨ���У����á����������ˡ������������ȣ�
��Һ��һ�־�һ���ȶ��Ļ���
��д��ѧ����ʽҪע��淶�ԣ�
����ˮ����ʹ�õľ�ˮ����ͨ���У����á����������ˡ������������ȣ�
��Һ��һ�־�һ���ȶ��Ļ���
��д��ѧ����ʽҪע��淶�ԣ�
����⣺��1������Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����Ԫ�أ����C��
�ڼ������Ȫˮ��Ӳˮ������ˮ�ļ����ǣ�ȡ�����������ˮ�������۲����������ĭ�϶࣬����ˮ�����û����ĭ����ĭ���٣���Ӳˮ��
���ȡ�����������ˮ�������۲����������ĭ�϶࣬����ˮ�����û����ĭ����ĭ���٣���Ӳˮ��
��2������ͼ1��֪����������ˮ����ʹ�õľ�ˮ�����У�ͨ�������ؽ��г�����ͨ��ɳ�˽��й��˵ȣ����AB��
����ʯ����ˮ��Ӧ�ܹ������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O=Ca��OH��2�����CaO+H2O=Ca��OH��2��
�ۻ�ѧ��Ӧǰ��Ԫ�ص�����䣬ԭ�ӵ�����䣬ԭ�ӵĸ������䣬�ɴ˿�֪��������ˮ��Ӧ�����ɴ������⣬������һ���������Ȼ��⣬�Ȼ���Ļ�ѧʽ��HCl�����HCl��
��3��ֲ���Ͳ�����ˮ�����ܺ�ˮ�γ���Һ�����ᡢ���ǡ�������ص�����������ˮ���ܺ�ˮ�γ���Һ�����A��
��4����ͨ�������£�ˮ�ܹ��ֽ�������������������Ӧ�Ļ�ѧ����ʽΪ��2H2O
2H2��+O2�������2H2O
2H2��+O2����
���ˮʱ�������������������в��������������븺�������������в������������������Ϊ��1��2�����1��2��
�ڼ������Ȫˮ��Ӳˮ������ˮ�ļ����ǣ�ȡ�����������ˮ�������۲����������ĭ�϶࣬����ˮ�����û����ĭ����ĭ���٣���Ӳˮ��
���ȡ�����������ˮ�������۲����������ĭ�϶࣬����ˮ�����û����ĭ����ĭ���٣���Ӳˮ��
��2������ͼ1��֪����������ˮ����ʹ�õľ�ˮ�����У�ͨ�������ؽ��г�����ͨ��ɳ�˽��й��˵ȣ����AB��
����ʯ����ˮ��Ӧ�ܹ������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O=Ca��OH��2�����CaO+H2O=Ca��OH��2��
�ۻ�ѧ��Ӧǰ��Ԫ�ص�����䣬ԭ�ӵ�����䣬ԭ�ӵĸ������䣬�ɴ˿�֪��������ˮ��Ӧ�����ɴ������⣬������һ���������Ȼ��⣬�Ȼ���Ļ�ѧʽ��HCl�����HCl��
��3��ֲ���Ͳ�����ˮ�����ܺ�ˮ�γ���Һ�����ᡢ���ǡ�������ص�����������ˮ���ܺ�ˮ�γ���Һ�����A��
��4����ͨ�������£�ˮ�ܹ��ֽ�������������������Ӧ�Ļ�ѧ����ʽΪ��2H2O
| ||
| ||
���ˮʱ�������������������в��������������븺�������������в������������������Ϊ��1��2�����1��2��
������������Ҫ���龻��ˮ�ķ�������ˮ��Ӳˮ�ļ��顢��ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע���IJ���һ�Ƿ�Ӧ���������Ļ�ѧʽҪ��ȷ��������ѭ�����غ㶨�ɣ�����д�ϱ�Ҫ�����������ǿ��Ƿ��С�����������

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ