��Ŀ����
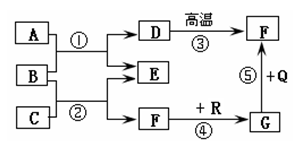
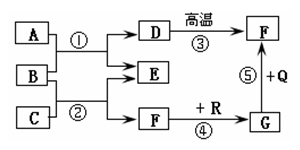
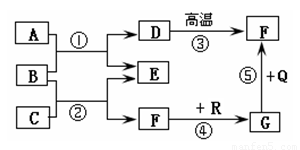
(6��)��ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����У���ͨ������£�F��G�����Ԫ����ͬ���������壬B�ǿ�����̼���Σ�Q��R���Ǻ�ɫ����(���ַ�Ӧ���������ֲ�����ȥ)���Իش��������⣺
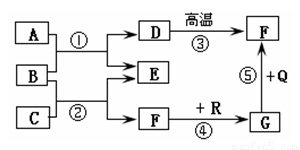
(1)���ƶ�D��G�Ļ�ѧʽΪ��D______ G______��
(2)��E�������г��õĵ�ζƷ��������, ��C�Ļ�ѧʽΪ_________����Ӧ�ٵĻ�ѧ����ʽΪ��
______________________________________
(3)��Ӧ���ڹ�ҵ�ϱ��㷺Ӧ���ڽ�����ұ����������������ұ�����������Ļ�ѧ����ʽΪ��
_____________________________________��
(4)��Ӧ��~����û���漰�Ļ�����Ӧ������_______________________________��
���𰸡�
(1)CaCO3��CO (2)HCl�� CaCl2+Na2CO3=CaCO3��+2NaCl
(3)3CO+Fe2O3������������2Fe+3CO2 (4)�û���Ӧ
����������

��ϰ��ϵ�д�
�����Ŀ