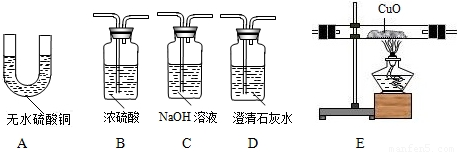
��Ŀ����
[ʵ��Ŀ��]ȷ��������Ʒ���Ƿ���CO
[��������]��Ȼ������Ҫ�ɷ��Ǽ��鼰��������Ͷ�����̼�ȣ�������������ɫ�����ᡢ��ʴ�ԣ����а�ȫ����ֵ�ߡ��ྻ��Ӧ�ù㷺���ŵ㣮�ڿ�������ȫȼ������CO2��H2O������ȫȼ��ʱ������CO��ˮ��֤��һ������ˮ����ͭ����ˮ����ͭ��һ�ְ�ɫ���壬��ˮ�����ͻ�����ɫ��
[ʵ������]

[����]
��1��Ҫ�ýϼķ����ռ���ը�ֳ���������Ʒ����ѡ��ʲô����
��2����ʵ��ʱ����������������˳����
��3��������ȷ��������Ʒ�к���CO������
��4���ӻ��������ĽǶȿ��ǣ�����Ϊ��ʵ���Ƿ����ȱ�ݣ�����У���θĽ���ʵ�飿������Ϊ��ʵ��û��ȱ�ݣ��������ⲻ�ػش𣩸Ľ��ķ�����
��2��ʵ��С���Ŀ���Ǽ����ֳ����������Ƿ���CO��ˮ����������Ҫ����ˮ����������ˮ��������ˮ����ͭ������һ����̼Ҫ��������ԭ����ͭ�Ƿ����ɶ�����̼��������˳������ͨ����ˮ����ͭ����ˮ�Ĵ��ڣ�Ȼ��������������Һ��ȥ������̼����Ũ����������壬ͨ�����ȵ�����ͭ�������ɵ�����ͨ������ʯ��ˮ��
��3����ȷ��������Ʒ�к���CO�������� E�к�ɫ��ĩ��죬D��ʯ��ˮ����ǣ�װ��D�з�Ӧ�ķ���ʽ�� CO2+Ca��OH��2�TCaCO3��+H2O��
��4����Ϊһ����̼�ж����ӻ��������ĽǶȿ��ǣ�Ҫ��β�����д�����
�ʴ�Ϊ����1�������ռ���
��2��A��C��B��E��D��
��3��E�к�ɫ��ĩ��죬D��ʯ��ˮ����ǣ� CO2+Ca��OH��2�TCaCO3��+H2O��
��4����D���ڴ��������ռ����ȼ��

| |||||||||||||||||||||||||||||||||||||||||||||