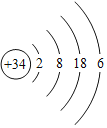
��Ŀ����
��֪��2Na2O2+2CO2��2Na2CO3+O2��2Na2O2+2H2O��4NaOH+O2����Ϊ��֤���������ƣ�Na2O2��������ں�����ߺ�DZˮͧ������������ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���̽�����������̨������ȥ��
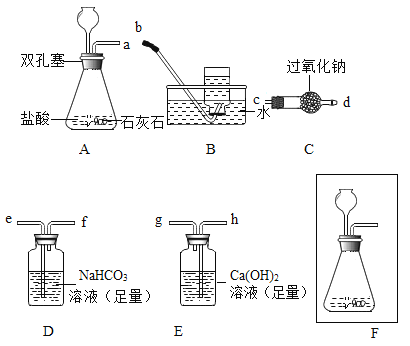
��1��A װ������������Ӧ�Ļ�ѧ����ʽΪ________________��
��2���뽫��ͼF������Aװ�õij���©���Ͳ������ܲ�������_______________��
��3��Dװ�õ�������________________��Eװ���п��ܷ������йط�Ӧ�Ļ�ѧ����ʽΪ_______________��
��4��Ϊ�˴ﵽʵ���Ŀ�ģ�����װ�õ��ܽӿ���ȷ������˳��Ϊ��a ��_____________��________________��________________��________________��________________��________________�� b��
��5�������ɲ���Bװ���ռ����ô����ǵ�ľ�����飬������������������Щ���ʣ�д������_____��_____
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д����г�ȥ���ʣ��������ķ����У���ȷ���ǣ�������
ѡ�� | ���� | ���� | ���ӷ��� |
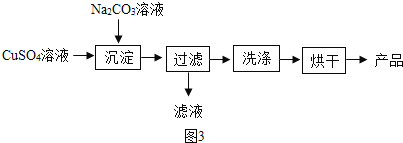
A | H2 | HCl | ͨ�����͵�NaCO3��Һ |
B | KNO3���� | NaCl | �������Ƴɱ�����Һ�������ᾧ�����ˣ�ϴ�Ӻ�� |
C | NaCl���� | Na2CO3 | ���Թ���ϡ���ᣬ�����ᾧ |
D | KCl��Һ | K2SO4 | ������Ba �� NO3��2��Һ������ |
A.A B.B C.C D.D

 ��100%��ƫ�ߣ�����ܵ�ԭ��Ϊ________������ţ�
��100%��ƫ�ߣ�����ܵ�ԭ��Ϊ________������ţ�
 MgO + H2O��̼��Ƹ����·ֽ⡣ij��ȤС��Ϊ�ⶨ���и��ɷֵ�����������ȡ12.9gˮ����Ʒ���ȣ����ȹ�����ʣ���������������ʱ��ı仯����ͼ��ʾ�����ڼ���һ��ʱ���ʣ�������жϣ�����˵��������ǣ� ��
MgO + H2O��̼��Ƹ����·ֽ⡣ij��ȤС��Ϊ�ⶨ���и��ɷֵ�����������ȡ12.9gˮ����Ʒ���ȣ����ȹ�����ʣ���������������ʱ��ı仯����ͼ��ʾ�����ڼ���һ��ʱ���ʣ�������жϣ�����˵��������ǣ� ��