��Ŀ����
�ش��������⣮
��1��20����80�������������һ����ˮ���͡���ƭ�֣�ƭ�������߳ƣ�ֻҪ��ˮ�м���һЩ�����ء�������ˮ�Ϳ��Ա����ȼ�յ����ͣ���Ҫ��C��HԪ�أ����������ˮ���ܱ�����͵�ԭ��______��
��2����A��B������ʢ��20mL����ˮ��С�ձ��У��ֱ�����̪��Һ3�Σ���ȡһ��С�ձ�C������Լ5mLŨ��ˮ����ˮ�ʼ��ԣ�����һ�����ձ���סA��C����С�ձ����ձ�B���ڴ��ձ��⣨��ͼ���������Ӻ۲쵽�������ǣ��ձ�A______���ձ�B______����������˵����ʲô�����ӷ��ӵĽǶȻش�______��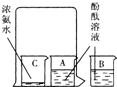
��1��20����80�������������һ����ˮ���͡���ƭ�֣�ƭ�������߳ƣ�ֻҪ��ˮ�м���һЩ�����ء�������ˮ�Ϳ��Ա����ȼ�յ����ͣ���Ҫ��C��HԪ�أ����������ˮ���ܱ�����͵�ԭ��______��
��2����A��B������ʢ��20mL����ˮ��С�ձ��У��ֱ�����̪��Һ3�Σ���ȡһ��С�ձ�C������Լ5mLŨ��ˮ����ˮ�ʼ��ԣ�����һ�����ձ���סA��C����С�ձ����ձ�B���ڴ��ձ��⣨��ͼ���������Ӻ۲쵽�������ǣ��ձ�A______���ձ�B______����������˵����ʲô�����ӷ��ӵĽǶȻش�______��

��1�����������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ�ص�����䣻ˮ�����⡢��Ԫ����ɵģ���������Ҫ����̼����Ԫ����ɵģ�ˮ�в���̼Ԫ�أ�����ˮ���ܱ�����ͣ�
��2��C�ձ��е�Ũ��ˮ���лӷ��ԣ��ӷ������İ����˶���A�ձ�������ˮ�γɵİ�ˮ�ʼ��ԣ�ʹ��̪��Һ��죻��B�ձ����ڴ��ձ��⣬�����еķ�̪��Һ���ܽӴ����������ʣ����Բ���죬��Ϊ��ɫ��A��B�����ձ��е�����û�л�ϣ���B�ձ��еķ�̪��Һȴ����ˣ�˵���������ڲ����˶��ģ�
�ʴ�Ϊ����1�����������غ㶨�ɣ���Ӧǰ��Ԫ�ص�����䣬��������̼Ԫ�أ���ˮ��û��̼Ԫ�أ�
��2����Һ����ɫ��Ϊ��ɫ����Һ��Ϊ��ɫ���ձ�C�еİ������˶����ձ�A�У�
��2��C�ձ��е�Ũ��ˮ���лӷ��ԣ��ӷ������İ����˶���A�ձ�������ˮ�γɵİ�ˮ�ʼ��ԣ�ʹ��̪��Һ��죻��B�ձ����ڴ��ձ��⣬�����еķ�̪��Һ���ܽӴ����������ʣ����Բ���죬��Ϊ��ɫ��A��B�����ձ��е�����û�л�ϣ���B�ձ��еķ�̪��Һȴ����ˣ�˵���������ڲ����˶��ģ�
�ʴ�Ϊ����1�����������غ㶨�ɣ���Ӧǰ��Ԫ�ص�����䣬��������̼Ԫ�أ���ˮ��û��̼Ԫ�أ�
��2����Һ����ɫ��Ϊ��ɫ����Һ��Ϊ��ɫ���ձ�C�еİ������˶����ձ�A�У�

��ϰ��ϵ�д�
�����Ŀ
��ͨ��ͭ����ͭ��п��ɣ��㷺���������ġ��ܲĵȣ�Ҳ���������е�����Ϊ�ⶨ��ͭ��ͭ������������ȡ��Ʒ10g�����Ĵ������м���ϡ����ʹ֮��ַ�Ӧ��ʵ�����ݼ�¼���±���
����������ݣ��ش��������⣺
��1����ͭ��ͭ����������Ϊ
��2��ǡ����ȫ��Ӧʱ����Һ�����ʵ����������ǣ�
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ���������/g | 10 | 10 | 10 | 10 |
| ʣ����������/g | 8.7 | 7.4 | 7 | 7 |
��1����ͭ��ͭ����������Ϊ
��2��ǡ����ȫ��Ӧʱ����Һ�����ʵ����������ǣ�
��Һ�����������ϢϢ��أ���Һ���������ճ�����ͻ�ѧʵ���еij����������±���������Һ�Ͱ�ˮ���ܶ��������ʵ������������ձ���20�棩��
����ϸ������ش��������⣺
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ��� �����������䣩����ˮ���ܶ��� ��������С�䣩
��2��ȡ12%��������Һ100g���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������
Ӧ 100g������ڡ�С�ڻ���ڣ���
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ����� mL��������0.1����
| ��Һ�����ʵ���������/% | 4 | 12 | 16 | 24 | 28 |
| ������Һ���ܶ�/g/mL | 1.02 | 1.08 | 1.11 | 1.17 | 1.20 |
| ��ˮ���ܶ�/g/mL | 0.98 | 0.95 | 0.94 | 0.91 | 0.90 |
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ���
��2��ȡ12%��������Һ100g���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������
Ӧ
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ�����
