��Ŀ����
��֪20��CʱCa (OH) 2���ܽ��Ϊ0.165g,����20��Cʱ����7.4g���ʵı��ͳ���ʯ��ˮ�������:
(1)��������ʯ��ˮ������Ϊ____________g (��ȷ��1g)��
(2)����������ʯ��ˮ��ͨ��CO2,�����������ﵽ���ֵʱ��ͨ��CO2������Ϊ����?____ (д ���������)
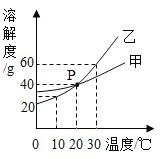
(3)��֪:  �� Ca (HCO3) 2 ������ˮ������ͼ�л���ͨ��CO2�����г��������ı仯����_____��
�� Ca (HCO3) 2 ������ˮ������ͼ�л���ͨ��CO2�����г��������ı仯����_____��
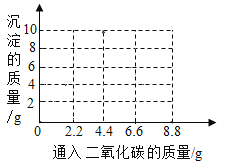
(4)��ȡһ�����ı��ͳ���ʯ��ˮ��ͨ��һ��ʱ���CO2,��Ӧ������������������±�
���� | Ca (OH) 2 | CO2 | CaCO3 | X | H2O |
����/g | 14.8 | 13.2 | 10 | a | 1.8 |
��a=______________����Ӧ�Ļ�ѧ����ʽΪ______________________________��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�±��г��˳�ȥ�����������������ʵķ�����������ȷ����()
ѡ�� | ���� | ���� | ��ȥ���ʵķ��� |
A | CuSO4��Һ | H2SO4 | �ӹ�����CuO,���� |
B | KNO3 | K2SO4 | ������BaCl2��Һ�����ˡ����� |
C | NaCl | ��ɳ | ��ˮ�ܽ⡢���ˡ�ϴ�ӡ����� |
D | NH3 | H2O | ͨ��Ũ���� |
A.A B.B C.C D.D