��Ŀ����
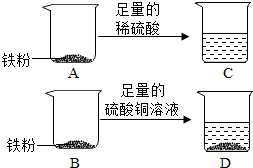 ��2012?������ʵ������һ�����������ۣ�С��Ϊ��̽�������Ļ�ѧ���ʲ��ⶨ�������ԭ���������������ͼ��ʵ�飮
��2012?������ʵ������һ�����������ۣ�С��Ϊ��̽�������Ļ�ѧ���ʲ��ⶨ�������ԭ���������������ͼ��ʵ�飮[ʵ�鲽��]
��ȡ��������ͬΪw�˵����ۣ��ֱ�����������ȵ�A��B�ձ��У�
����װ�����۵�A�ձ��м���50��������ϡ�����װ�����۵�B�ձ��м���50������������ͭ��Һ��
�۳�ַ�Ӧ����г�����C�ձ����ձ������ʵ�������Ϊm�ˣ�D�ձ����ձ������ʵ�������Ϊn�ˣ�
��1����д��C�ձ��з�����Ӧ�Ļ�ѧ����ʽ
Fe+H2SO4�TFeSO4+H2��
Fe+H2SO4�TFeSO4+H2��
��ͨ��C�ձ��з����Ļ�ѧ��Ӧ�����ܵó��Ľ����������û������е���
�����û������е���
����2��D�ձ��з�����Ӧ�Ļ�ѧ����ʽΪ
CuSO4+Fe�TFeSO4+Cu
CuSO4+Fe�TFeSO4+Cu
���˷�Ӧ����֤�����Ľ�����Ա�ͭǿ
ǿ
���ǿ��������������3���������ԭ�������ı���ʽΪ
| 2w |
| n-m |
| 2w |
| n-m |
��������1������������ϡ���ᷴӦ��������������������д����ʽ���õ����ۣ�
��2���������Ļ�Ա�ͭǿ�����������û�������ͭ�е�ͭ�������
��3������D�ձ��з�Ӧ�����ʵ���������������غ㶨������ձ���������������������ϡ����ķ�Ӧ������������������������������ԭ��������
��2���������Ļ�Ա�ͭǿ�����������û�������ͭ�е�ͭ�������
��3������D�ձ��з�Ӧ�����ʵ���������������غ㶨������ձ���������������������ϡ����ķ�Ӧ������������������������������ԭ��������
����⣺��1�����Ļ������ǰ������������ϡ���ᷴӦ���������������������䷽��ʽΪFe+H2SO4�TFeSO4+H2����
��2���ɽ������˳�����֪���Ļ�Ա�ͭǿ�����������û�������ͭ�е�ͭ���䷽��ʽΪCuSO4+Fe�TFeSO4+Cu��
��3����ΪD�ձ��з�Ӧ�����ʵ���������n�ˣ������������غ㶨�ɿ�֪��Ӧ��D�ձ������ʵ��������ǣ�50+w���ˣ������ձ�������Ϊ��n��-��50+w���ˣ���������ϡ����ķ�Ӧ����������������50��+w��-{m��-[n��-��50+w����]}=��n-m����
���������ԭ��������x
Fe+H2SO4�TFeSO4+H2��
x 2
w�� ��n-m����
=
x=
�ʴ�Ϊ����1��Fe+H2SO4�TFeSO4+H2���������û������е��⣻��2��CuSO4+Fe�TFeSO4+Cu��ǿ����3��
��
��2���ɽ������˳�����֪���Ļ�Ա�ͭǿ�����������û�������ͭ�е�ͭ���䷽��ʽΪCuSO4+Fe�TFeSO4+Cu��
��3����ΪD�ձ��з�Ӧ�����ʵ���������n�ˣ������������غ㶨�ɿ�֪��Ӧ��D�ձ������ʵ��������ǣ�50+w���ˣ������ձ�������Ϊ��n��-��50+w���ˣ���������ϡ����ķ�Ӧ����������������50��+w��-{m��-[n��-��50+w����]}=��n-m����
���������ԭ��������x
Fe+H2SO4�TFeSO4+H2��
x 2
w�� ��n-m����
| x |
| 2 |
| w |
| (n-m)�� |
x=
| 2w |
| n-m |
�ʴ�Ϊ����1��Fe+H2SO4�TFeSO4+H2���������û������е��⣻��2��CuSO4+Fe�TFeSO4+Cu��ǿ����3��
| 2w |
| n-m |
�����������Ƕ����������û���Ӧ�Ŀ��飬���ս������˳��������Լ�Ӧ�ã��Լ���ѧ����ʽ���йؼ����ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
