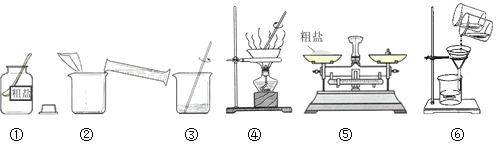
��Ŀ����
����Ŀ���������к�40%��60%Al2O3��1%��15%SiO2��7%��30%Fe2O3��������������ȡ�������Ĺ�����ͼ��
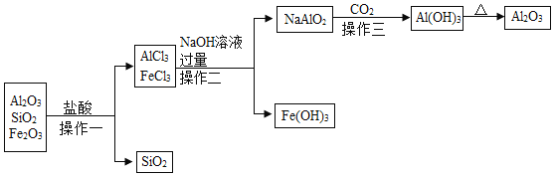
(��֪��SiO2������ˮ��NaAlO2����ˮ��Al(OH)3+NaOH=NaAlO2+2H2O��2NaAlO2+CO2+3H2O=2Al(OH)3��+Na2CO3)
(1)����һ�������������������������ж�Ҫ���õķ��뷽����______��
(2)д������һ������Ӧ�Ļ�ѧ����ʽ______��
(3)������������ȡAl2O3ʱ����������Al2O3-AlCl3-NaAlO2-Al(OH)3-Al2O3����ת�����̣��������������Ŀ����ʲô��______��
(4)��������NaOH��Һ������Ŀ����______��
���𰸡����� Al2O3+6HCl=2AlCl3+3H2O Fe2O3+6HCl=2FeCl3+3H2O ��������������������ñȽϴ����������� ʹAlCl3ת��Ϊ�����Ե�NaAlO2���Ӷ���Fe(OH)3����
��������
(1)����һ�������������������������ж������������Թ�����Һ��ķ��룬���ڹ��˲�����������ˣ�
(2)����һ�У������������ᷴӦ�����Ȼ�����ˮ�������������ᷴӦ�����Ȼ�����ˮ�����Al2O3+6HCl=2AlCl3+3H2O��Fe2O3+6HCl=2FeCl3+3H2O��
(3)�ɹ�������ͼ��֪��������������ȡAl2O3ʱ����������Al2O3-AlCl3-NaAlO2-Al(OH)3-Al2O3����ת�����̣���������Ŀ���ǽ�������������������ñȽϴ������������������������������������ñȽϴ�������������
(4)�������У��Ȼ������������Ʒ�Ӧ����NaAlO2���Ӷ���Fe(OH)3���룬���Բ�������NaOH��Һ������Ŀ����ʹAlCl3ת��Ϊ�����Ե�NaAlO2���Ӷ���Fe(OH)3���룻���ʹAlCl3ת��Ϊ�����Ե�NaAlO2���Ӷ���Fe(OH)3���롣

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ��С����̽����Ļ�ѧ����ʱ,��ϡ������뵽����������Һ�У�����ط��������ݲ���������Ϊ����������Һ�Ѿ����ʡ�����������Һ���ʵ�ԭ����______���û�ѧ����ʽ��ʾ����
С��Ա��ʺ������������Һ�е����ʳɷ����������ֲ��룺
����һ���������Ʋ��ֱ��ʣ����ʺ����Һ�к���![]() ��
��![]()
���������������ȫ�����ʣ����ʺ����Һ��ֻ����![]()
��ʵ����ƣ�Ϊ����֤����һ��С����������·�����ȡ�������ʺ������������Һ��Ʒ���Թ��У��μӼ�����ɫ��̪��Һ���۲���Һ��ɫ���ɫ��˵����Һ�к����������ƣ��������Ʋ��ֱ��ʡ�С����ΪС���ʵ�鷽����������������______��
��ʵ������ۣ�С���������ʵ�鷽����֤����������������������ʵ�鱨�棺
ʵ�鲽�� | ʵ������ | ���� |
ʵ��1��ȡ��������������Һ��Ʒ���Թ��У������еμӹ�������������Һ���� | �а�ɫ�������� | �������ȷ |
ʵ��2����ʵ��1�Թ��еĻ������ˣ�______ | _______ |
ʵ��1��Ӧ�Ļ�ѧ����ʽ��______��