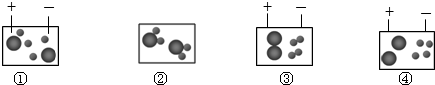
��Ŀ����
����Ԫ�ؿ��������������,��ѡ���±��е�Ԫ�ػش��������⡣
��1�����û�ѧ���ű�ʾ��ÿ��ֻдһ�֣���
�پ��п�ȼ�Ե����嵥�� ����ʹ����ʯ��ˮ����ǵ�����
���������к�����ߵ�Ԫ�� �ܺ�������Ԫ��������
��2����Ȼ����ú̿��ʯ�͵���Դ��Ⱦ���ʹ�ð�ȫ����ֵ�ߡ��ྻ�����ơ���Ȼ������Ҫ�ɷ��Ǽ��飬���������������顢���顢������̼��һ����̼�ȡ��ݴ˻ش�
����Ȼ������ ����������������
�ڼ���ȼ��ʱ�����Ļ�ѧ��Ӧ����ʾ��ͼ����ͼ��ʾ�����У� ��ʾ̼ԭ�ӣ�
��ʾ̼ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӡ�
��ʾ��ԭ�ӡ�

A B C D
����A�з���Ϊ������ӣ�1����������к��� ��ԭ�ӡ�
��������̼����Ԫ��������Ϊ ��
������ͼʾ��Ϣ��д������ȼ�յĻ�ѧ����ʽ ��
����֪���飨C2H6����ȼ�ղ���������ȼ�ղ�����ȫ��ͬ�����������ļ����������ȼ�գ� ������顱�����顱������������Ķ�����̼��
| Ԫ������ | �� | ̼ | �� | �� |
��1�����û�ѧ���ű�ʾ��ÿ��ֻдһ�֣���
�پ��п�ȼ�Ե����嵥�� ����ʹ����ʯ��ˮ����ǵ�����
���������к�����ߵ�Ԫ�� �ܺ�������Ԫ��������
��2����Ȼ����ú̿��ʯ�͵���Դ��Ⱦ���ʹ�ð�ȫ����ֵ�ߡ��ྻ�����ơ���Ȼ������Ҫ�ɷ��Ǽ��飬���������������顢���顢������̼��һ����̼�ȡ��ݴ˻ش�
����Ȼ������ ����������������
�ڼ���ȼ��ʱ�����Ļ�ѧ��Ӧ����ʾ��ͼ����ͼ��ʾ�����У�
 ��ʾ̼ԭ�ӣ�
��ʾ̼ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӡ�
��ʾ��ԭ�ӡ�
A B C D
����A�з���Ϊ������ӣ�1����������к��� ��ԭ�ӡ�
��������̼����Ԫ��������Ϊ ��
������ͼʾ��Ϣ��д������ȼ�յĻ�ѧ����ʽ ��
����֪���飨C2H6����ȼ�ղ���������ȼ�ղ�����ȫ��ͬ�����������ļ����������ȼ�գ� ������顱�����顱������������Ķ�����̼��
��1���� H2 �� CO2 ��O ��OH- ��CO32-
��2���ٻ����
�ڣ���5 ���� 3:1 �� 12:4����CH4+2O2
 CO2+2H2O
CO2+2H2O������
�����������2��������֪����Ȼ���к��ж������ʣ����������ڻ����ڣ��۲������ӽṹͼ�����Կ���һ�������������һ��̼ԭ�����ĸ���ԭ�ӹ��ɵģ��ʹ�5�����ӡ���������̼����Ԫ��������Ϊ12��1��4=12��4��������ͼʾ��Ϣ������ȼ�յķ�Ӧ��Ϊ����CH4����������O2����Ӧ����Ϊ��ȼ��������Ϊ������̼CO2��ˮH2O���ʷ���ʽдΪCH4+2O2
 CO2+2H2O �۹۲컯ѧʽ�ɿ����������е�̼Ԫ�����������ȼ���ߵö࣬��Щ̼Ԫ��ȼ�պ�ȫ��ת��Ϊ������̼���ʵ��������������ʣ���ȫȼ�����ɶ�����̼����������ࡣ
CO2+2H2O �۹۲컯ѧʽ�ɿ����������е�̼Ԫ�����������ȼ���ߵö࣬��Щ̼Ԫ��ȼ�պ�ȫ��ת��Ϊ������̼���ʵ��������������ʣ���ȫȼ�����ɶ�����̼����������ࡣ
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
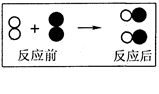
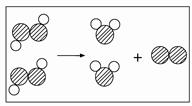

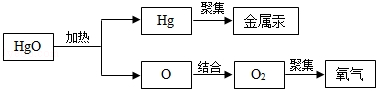



 2C+3D����A�Ļ�ѧʽΪ ��A��B��C��D��������������� ������ĸ����
2C+3D����A�Ļ�ѧʽΪ ��A��B��C��D��������������� ������ĸ����