��Ŀ����
������ʾ�������Ƶ�������������Ϊ5%��H2O2��Һ�����ȵ�80��ʱ�����н϶���������������ͬ����5%��H2O2��Һ��������������¾ͻ�����������������Ӧ�ٶȿ죬����ʱ��̡�

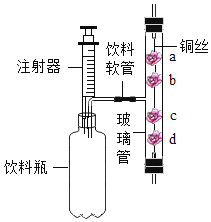
��1��С����ͼ��װ�ý���ʵ�飬���Թ����д������ݳ���ʱ����������ǵ�ľ����ľ����δ��ȼ��Ϊ�ˣ�������ͼ��װ���ռ����壬���ô����ǵ�ľ�����飬ľ����ȼ����ôͼ��ʵ���д�����ľ��δ��ȼ��ԭ���� ��
��2��С�����ô���ʹH2O2��Һ�ֽ���ȡ������ͼ��������Ƶ����巢��װ�ã�����ָ��һ������
��
��3��������ͬ����5%��H2O2��Һ��ͼ�����߱�ʾ���ȷֽ���ȡ���������ߣ������ڸ�ͼ����ʵ�������ô�����ȡ�����Ĵ������ߣ� ���ٶ����ַ���H2O2����ȫ�ֽ⣻�����ڴ���ֽ�ϣ���
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


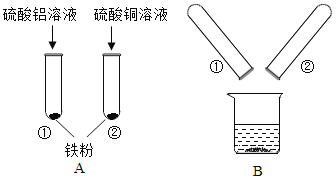
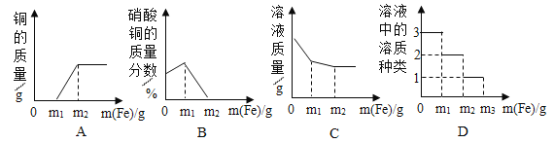
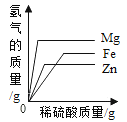 �ֱ�����ͬ������þ�ۡ����ۡ�п���м����Ũ��ϡ����
�ֱ�����ͬ������þ�ۡ����ۡ�п���м����Ũ��ϡ����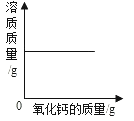 һ���¶��£��͵�����������Һ�м��������ƹ���
һ���¶��£��͵�����������Һ�м��������ƹ��� ��һ���������м���ϡ����
��һ���������м���ϡ���� ��һ������������Һ�м���ͭ��
��һ������������Һ�м���ͭ��