��Ŀ����
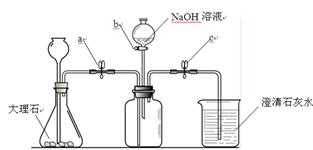
(5�֣�ij��ѧС���ͬѧ����ij�����ϵ���ɽ��з����о���������ʾ������ֻ��C��H����Ԫ�أ����������������ͼ��ʾ��ʵ��װ�á�Ŀ����ͨ�������й����ݣ������������Ԫ�صĺ�����ͼ����ĸA��G����ʾװ����ţ���

�����ʵ��װ�ã��ش��������⣺
(1) Aװ����ȡ�������л�������CO2��ˮ������ΪʹDװ�������������ڴ�����ȼ�գ����ڳ�ȥCO2��װ��Ϊ ����װ����ţ�����Ӧ�Ļ�ѧ����ʽΪ ��
(2)���������װ��E�е��Լ�Ϊ ��
(3)��ʯ�ҵijɷ����������ƺ������ƣ�Gװ�õ������� ��

�����ʵ��װ�ã��ش��������⣺
(1) Aװ����ȡ�������л�������CO2��ˮ������ΪʹDװ�������������ڴ�����ȼ�գ����ڳ�ȥCO2��װ��Ϊ ����װ����ţ�����Ӧ�Ļ�ѧ����ʽΪ ��
(2)���������װ��E�е��Լ�Ϊ ��
(3)��ʯ�ҵijɷ����������ƺ������ƣ�Gװ�õ������� ��
��1��B 2NaOH+CO2===Na2CO3+ H2O
��2��Ũ����
��3�����տ����еĶ�����̼��ʹʵ������ȷ��
��2��Ũ����
��3�����տ����еĶ�����̼��ʹʵ������ȷ��
����������������е�֪ʶ���з����������������������̼��Ӧ����̼���ƺ�ˮ���������������Ƴ�ȥ������̼��Ũ���������ˮ�ԣ������ڸ���ijЩ���塣
��1��Ҫ��ȥ�����е�ˮ�����Ͷ�����̼��Ӧ�ȳ�ȥ������̼�����ȥˮ�����ȳ�ȥˮ������������������Һ��ȥ������̼��ͬʱ������Һ�����������ֻ����ˮ����Ҫ�ȳ�ȥ������̼����Bװ��Ҫװ����������Һ���Գ�ȥ������̼����Ӧ�Ļ�ѧ����ʽΪ2NaOH+CO2===Na2CO3+ H2O��
��2������ȼ�������ɶ�����̼��ˮ��Ҫ�ⶨ̼����Ԫ�صĺ���������Ҫ�ⶨ���ɵ�ˮ�Ͷ�����̼��������Ҳ����Ҫ�ֱ�����ˮ�Ͷ�����̼�����ڴ�����������Һ�г����������ֻ����ˮ����Ӱ�쵽ʵ��Ľ������Ҫ������ˮ�������ն�����̼����Eʢ��Ũ���ᡣ
��3��Ҫ��ⶨ��������еĸ�Ԫ�صĺ�������Ҫ��ȥ�������ųɷ֣�ʹ�ü�ʯ���ܽ������е�ˮ�����Ͷ�����̼��������ֹ������F��ʹʵ������ȷ��
��������ʵ���Ǹ���������ʵ�飬��Ҫ֪��ˮ�Ͷ�����̼��������Ҳ���Ƿֱ�����ˮ�Ͷ�����̼����������Ҫ�ֱ����ʱ���൱�ڼ��飩������Ҫˮ��ǰ��������̼�ںͳ���ǡ���෴��

��ϰ��ϵ�д�
�����Ŀ
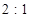 �����Լ�ʵ����������������������������Դ���2��1�������һ���֣�����Ϊ���������в���ȡ���ǣ� ��
�����Լ�ʵ����������������������������Դ���2��1�������һ���֣�����Ϊ���������в���ȡ���ǣ� ��