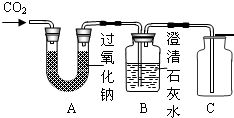
��Ŀ����
32���������ߺš��ɴ��ijɹ��������ҹ�������ҵ����һ��̱���������������ȿ�������ɴ���DZˮͧ�е��������������磺�������ƣ�Na2O2���ڳ����������˺����Ķ�����̼��Ӧ������������ѧ����ʽΪ��2Na2O2+2CO2�T2Na2CO3+O2��Ϊ����֤�÷�Ӧ��������������ij��ȤС���ͬѧ���������ͼ��ʾ��ʵ�飮

��1������Bƿ�����������
��2��Bƿװ����������ʯ��ˮ�����������
��3��Cװ�õ�������

��1������Bƿ�����������
�����Ͷ�����̼
����2��Bƿװ����������ʯ��ˮ�����������
��ȥδ��Ӧ���Ķ�����̼
���÷�Ӧ�Ļ�ѧ����ʽΪCO2+Ca��OH��2=CaCO3��+H2O
����3��Cװ�õ�������
�ռ�����
��Ϊ�˴ﵽ����ʵ��Ŀ�ģ���������ʵ�����������������ǵ�Сľ�����뼯��ƿ�У�
���������ڼ��������̼����ʱ�����ó����ʯ��ˮ�����У��ڼ�������ʱ�����ô����ǵ�ľ�������У��������ʯ��ˮ�����˵���Ƕ�����̼���������ǵ�ľ����ȼ��˵����������������
����⣺��1���������̼����������Ʒ�Ӧ����������������װ��B�п����е�����Ϊ�����Ͷ�����̼��
��2��Bװ���еij���ʯ��ˮ���ɼ���B���Ƿ��ж�����̼��Ҳ�����������Ķ�����̼��
��3��װ��C���������ռ��������������ô����ǵ�ľ�������飬�ռ��������Ƿ���������
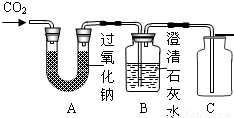
�ʴ�Ϊ��
��1������������̼
��2����ȥδ��Ӧ���Ķ�����̼��CO2+Ca��OH��2=CaCO3��+H2O
��3���ռ��������������ǵ�Сľ�����뼯��ƿ��
��2��Bװ���еij���ʯ��ˮ���ɼ���B���Ƿ��ж�����̼��Ҳ�����������Ķ�����̼��
��3��װ��C���������ռ��������������ô����ǵ�ľ�������飬�ռ��������Ƿ���������
�ʴ�Ϊ��
��1������������̼
��2����ȥδ��Ӧ���Ķ�����̼��CO2+Ca��OH��2=CaCO3��+H2O
��3���ռ��������������ǵ�Сľ�����뼯��ƿ��
��������������������ղ����Ķ�����̼���壬���������ں�����ҵ�л���ҵ�о����õ�������ƽ������ж�����̼�ĺ������ֿɵõ����������������

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
�����Ŀ