��Ŀ����
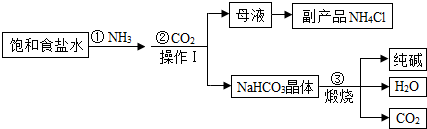
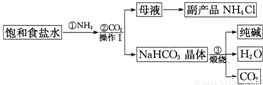
�ҹ���ѧ�Һ�°�Ľ��˹���Ĵ����������գ��������̿ɼ�Ҫ��ʾ���£�

��1��д�����������һ����;��
��2��д������۷�Ӧ�Ļ�ѧ����ʽ��
��3����������������
��4������I��������
��5����ҵ���õ�ⱥ��ʳ��ˮ�ķ�������ȡ�������ơ��������������÷�Ӧ�Ļ�ѧ����ʽΪ��

��1��д�����������һ����;��
��������ϴ�Ӽ�
��������ϴ�Ӽ�
����2��д������۷�Ӧ�Ļ�ѧ����ʽ��
2NaHCO3
Na2CO3+CO2��+H2O
| ||
2NaHCO3
Na2CO3+CO2��+H2O
��������Ӧ��������
| ||
�ֽ�
�ֽ�
��Ӧ����3����������������
������̼
������̼
��ѭ��ʹ�ã����ŵ��Ǽ�������ЧӦ�����ԭ�ϵ�������
���ԭ�ϵ�������
����4������I��������
����
����
���������ڸò����е�����������
����
����5����ҵ���õ�ⱥ��ʳ��ˮ�ķ�������ȡ�������ơ��������������÷�Ӧ�Ļ�ѧ����ʽΪ��
2NaCl+2H2O
2NaOH+Cl2��+H2��
| ||
2NaCl+2H2O
2NaOH+Cl2��+H2��
��
| ||
��������1�����ݴ������;������
��2������̼�����Ƽ�������̼���ơ�������̼��ˮ��ϻ�ѧ����ʽ����д������
��3�����ݹ������̿��Կ���������̼��ѭ��ʹ�ã�
��4�����ݹ��˵�ԭ���Լ������������÷�����
��5�����ݵ�ⱥ��ʳ��ˮ����ȡ�������ơ�������������ϻ�ѧ����ʽ����д������
��2������̼�����Ƽ�������̼���ơ�������̼��ˮ��ϻ�ѧ����ʽ����д������
��3�����ݹ������̿��Կ���������̼��ѭ��ʹ�ã�
��4�����ݹ��˵�ԭ���Լ������������÷�����
��5�����ݵ�ⱥ��ʳ��ˮ����ȡ�������ơ�������������ϻ�ѧ����ʽ����д������
����⣺��1���������������ϴ�Ӽ����Ʋ�������ֽ��ȥ�۷ۡ�ӡȾ�ȣ�
��2��̼�����Ƽ��ȷֽ�����̼���ơ�������̼��ˮ����Ӧ�Ļ�ѧ����ʽ��2NaHCO3
Na2CO3+CO2��+H2O���˷�Ӧ�ķ�Ӧ����һ�֣������������֣����ڷֽⷴӦ��
��3���������������̿��Կ���������̼��ѭ��ʹ�ã����ŵ��Ǽ�������ЧӦ�����ԭ�ϵ������ʣ�
��4������I�ǽ������Һ��ֿ��IJ������������ǹ��ˣ��������ڹ����е�������������
��5����ⱥ��ʳ��ˮ����ȡ�������ơ��������������÷�Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O
2NaOH+Cl2��+H2����
�ʴ�Ϊ����1����������ϴ�Ӽ���
��2��2NaHCO3
Na2CO3+CO2��+H2O���ֽ⣻
��3��������̼�����ԭ�ϵ������ʣ�
��4�����ˣ�������
��5��2NaCl+2H2O
2NaOH+Cl2��+H2����
��2��̼�����Ƽ��ȷֽ�����̼���ơ�������̼��ˮ����Ӧ�Ļ�ѧ����ʽ��2NaHCO3
| ||
��3���������������̿��Կ���������̼��ѭ��ʹ�ã����ŵ��Ǽ�������ЧӦ�����ԭ�ϵ������ʣ�
��4������I�ǽ������Һ��ֿ��IJ������������ǹ��ˣ��������ڹ����е�������������
��5����ⱥ��ʳ��ˮ����ȡ�������ơ��������������÷�Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O
| ||
�ʴ�Ϊ����1����������ϴ�Ӽ���
��2��2NaHCO3
| ||
��3��������̼�����ԭ�ϵ������ʣ�
��4�����ˣ�������
��5��2NaCl+2H2O
| ||
������������Ҫ�����˴������ȡ���й�֪ʶ�������˻�ѧ����ʽ����д�����˲����Լ������������õȣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ