��Ŀ����
����Ŀ���о���ѧϰ��̽��ʵ�����о��õ�NaOH�ı��ʳ̶�
���о��������ȳ�ȡ13��3g ��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14��6����ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȡ�
��1���۽�������ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ��
��д�±���������������С�����һλ��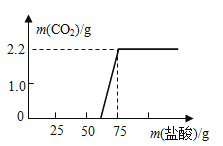
Na2CO3��������g | |
����NaOH������/g | |
NaOH�ı��ʳ̶� |
��2���ۼ���̽������ʵ���������NaOH��Ӧ���������������
��3���۷�������ݸ��ݡ���NaOH��Ӧ���������������������ͼ���㷢����ʲô���⣿
���𰸡�
��1��5.3��4.0��33.3%
��2��
ʵ���������NaOH��Ӧ���������������50g
��3��
NaOH����ȫ�кͺ�������Ϊ50g�����μ����ᣬΪʲôû����������CO2����
�������������ͼʾ��֪�����Ķ�����̼������Ϊ2.2g��
������2.2g������̼ʱ��Ҫ��̼����������x
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 2.2g
106��x=44��2.2g
x=5.g
����̼���Ƶ�����Ϊ5.3g���跴Ӧ���������Ƶ�����Ϊz
2NaOH+CO2�TNa2CO3+H2O
80 106
z 5.3g
80��z=106��.3g
y=4g
���������Ƶı��ʳ̶�Ϊ ![]()
[����̽��]û����������������Ϊ13.3g��5.3g=8g
��μӷ�Ӧ�Ȼ��������Ϊm
NaOH+HCl=NaCl+H2O
40 36.5
8g m
40��8g=36.5��m
m=7.3g��
���������=7.3g��14.6%=50g
=50g��
[��������]����ͼ�ο��Կ���NaOH����ȫ�кͺ�������Ϊ50g�����μ����ᣬΪʲôû����������CO2���壮
���Դ��ǣ�[�������]��
Na2CO3������/g | 5.3 |
����NaOH������/g | 4.0 |
NaOH�ı��ʳ̶ȣ�������������ʾ�� | 33.3% |
[����̽��]ʵ���������NaOH��Ӧ���������������50g��
[��������]��NaOH����ȫ�кͺ�������Ϊ50g�����μ����ᣬΪʲôû����������CO2���壮
���ʵ����������еμ����ᣬ������̼���Ʋ���������̼���壬���ݶ�����̼�����������ȷ��̼���Ƶ�������ͨ��ͼʾ���Ƿ��ָյμ�����ʱ��û�в���������̼���壬�Դ�Ϊͻ�ƿڷ�����ɣ�
�����㾫�����������⣬������Ҫ�˽���ݻ�ѧ��Ӧ����ʽ�ļ���(�����ʼ�������=ϵ������Է�������֮��)��

 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�����Ŀ��������������ֱ���þƾ��ƻ�����ȵ��ǣ� ��
����Ͳ ���Թ� ��ȼ�ճ� �ܼ���ƿ ���ձ� ����ƿ
A.�ڢ�
B.�ڢۢ�
C.�ݢ�
D.�ڢݢ�
����Ŀ������ʵ��Ŀ�Ķ�Ӧ��ʵ�鷽������ȷ���ǣ� ��
ʵ��Ŀ�� | ʵ�鷽�� | |
A | ���������̼��һ����̼ | �ֱ���������ͨ������ʯ��ˮ |
B | ����ˮ����������Һ | ����������̣��۲��Ƿ������� |
C | ��ˮ�Ϳ�Ȫˮ | �۲��Ƿ���� |
D | �ȽϿ������˺���������CO2�ĺ��� | �������ʯ��ˮ�����۲����� |
A.A
B.B
C.C
D.D
