��Ŀ����
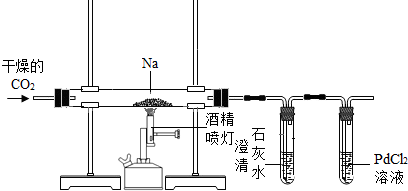
��֪�����ڽϸ��¶������������̼��Ӧ���÷�Ӧ��̼������ʲô����ͬ��ͬѧ����������ֲ��룺����Ϊ��C������Ϊ��CO������Ϊ��Na2CO3������Ϊ��CO��Na2CO3������Ϊ��NaHCO3��Ϊȷ���÷�Ӧ�ĺ�̼�����λͬѧ����ͼװ�ý���ʵ��̽����
��֪��CO����PdCl2���ɺ�ɫ��Pd�������ø÷�Ӧ���CO�Ƿ���ڡ��ش��������⣺
��֪��CO����PdCl2���ɺ�ɫ��Pd�������ø÷�Ӧ���CO�Ƿ���ڡ��ش��������⣺

��1������ʵ��Ϳ�֪����IJ����Ǵ���ģ���Ϊ�ò���Υ����___________________��
��2��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ_____________________________��
��3����ȡ������̼ʱ��Ϊ��ʹ�������̾��С��濪���ã������ͣ�����ص㣬Ӧѡ�õ�װ����__________��ѡ����ͼ�еġ�I������II����III������
��2��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ_____________________________��
��3����ȡ������̼ʱ��Ϊ��ʹ�������̾��С��濪���ã������ͣ�����ص㣬Ӧѡ�õ�װ����__________��ѡ����ͼ�еġ�I������II����III������

��4��ʵ��ʱӦ����װ�ƵIJ���ֱ��ͨCO2һ��ʱ�䣬װ����ʯ��ˮ���Թ��е�������______________________________��
��5��ʵ�鿪ʼ�۲쵽PdCl2��Һ���Թ����к�ɫ���ʲ������ҳ�ַ�Ӧ����ֱ���еĹ���ȫ���ܽ���ˮ��ȡ������Һ���������ʯ��ˮ���۲쵽�������ݴ˿�֪��
����λͬѧ�IJ�������ȷ�ģ���___________��
�����������̼��Ӧ�Ļ�ѧ����ʽΪ_____________________��
��5��ʵ�鿪ʼ�۲쵽PdCl2��Һ���Թ����к�ɫ���ʲ������ҳ�ַ�Ӧ����ֱ���еĹ���ȫ���ܽ���ˮ��ȡ������Һ���������ʯ��ˮ���۲쵽�������ݴ˿�֪��
����λͬѧ�IJ�������ȷ�ģ���___________��
�����������̼��Ӧ�Ļ�ѧ����ʽΪ_____________________��
��1�������غ㶨��
��2��CaCO3+2HCl===CaCl2+H2O+CO2��
��3����
��4������ʯ��ˮ�����
��5���ٶ�
��2CO2+2Na CO+Na2CO3
CO+Na2CO3
��2��CaCO3+2HCl===CaCl2+H2O+CO2��
��3����
��4������ʯ��ˮ�����
��5���ٶ�
��2CO2+2Na
 CO+Na2CO3
CO+Na2CO3
��ϰ��ϵ�д�
�����Ŀ




 [��������]��
[��������]��