��Ŀ����
�ܽ������ͼ�е��κ�һ�㶼��ʾ��Һ��һ���ض�״̬��ij�ֲ����ᾧˮ�Ĺ������ʵ��ܽ��������ͼ��ʾ���Ը���ͼ�ش��������⣺
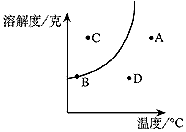
��1���������ߵı仯���ƣ���֪�����ʿ��������������еģ����ţ�����������
A. Ca(OH)2��B.NaCl��C. KNO3 D.CO2��������
��2��ͼ��A.B.C.D.�ĸ�״̬�У���Һ���ڲ�����״̬��������������״̬��ȶ�����������������
��3���������ʵ���Һ��D�㽵�µ�Bʱ�������������䣩����ʱ��Һ��Ũ�Ƚ�����������������. ����. ��С����
A. Ca(OH)2��B.NaCl��C. KNO3 D.CO2��������
��2��ͼ��A.B.C.D.�ĸ�״̬�У���Һ���ڲ�����״̬��������������״̬��ȶ�����������������
��3���������ʵ���Һ��D�㽵�µ�Bʱ�������������䣩����ʱ��Һ��Ũ�Ƚ�����������������. ����. ��С����
��1��C
��2��AD��C
��3������
��2��AD��C
��3������

��ϰ��ϵ�д�
�����Ŀ
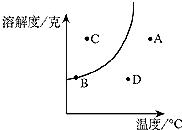 20���ܽ������ͼ�е��κ�һ�㶼��ʾ��Һ��һ���ض�״̬��ij�ֲ����ᾧˮ�Ĺ������ʵ��ܽ��������ͼ��ʾ���Ը���ͼ�ش��������⣺
20���ܽ������ͼ�е��κ�һ�㶼��ʾ��Һ��һ���ض�״̬��ij�ֲ����ᾧˮ�Ĺ������ʵ��ܽ��������ͼ��ʾ���Ը���ͼ�ش��������⣺ ��2010?���ݣ���ͼ��ijʵ��С���ͬѧ���Ƶ����ֹ������ʵ��ܽ������ͼ���������ͼʾ�ش����⣺
��2010?���ݣ���ͼ��ijʵ��С���ͬѧ���Ƶ����ֹ������ʵ��ܽ������ͼ���������ͼʾ�ش����⣺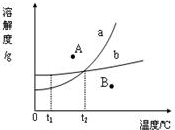 ��ͼ��ijʵ��С���ͬѧ���Ƶ����ֹ������ʵ��ܽ������ͼ���������ͼʾ�ش�����
��ͼ��ijʵ��С���ͬѧ���Ƶ����ֹ������ʵ��ܽ������ͼ���������ͼʾ�ش�����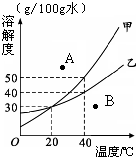 ��2011?��������ģ����ͼ��ijʵ��С����Ƶļ������ֹ������ʵ��ܽ������ͼ���������ͼʾ�ش����⣺
��2011?��������ģ����ͼ��ijʵ��С����Ƶļ������ֹ������ʵ��ܽ������ͼ���������ͼʾ�ش����⣺ ��ͼ��ijʵ��С���ͬѧ���Ƶ����ֹ������ʵ��ܽ������ͼ���������ͼʾ�ش����⣺
��ͼ��ijʵ��С���ͬѧ���Ƶ����ֹ������ʵ��ܽ������ͼ���������ͼʾ�ش����⣺