��Ŀ����
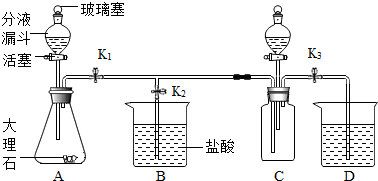 ��2012?��̨��һģ����ѧ��ȤС������ͼ��ʾװ�ý�������ʵ�飮
��2012?��̨��һģ����ѧ��ȤС������ͼ��ʾװ�ý�������ʵ�飮��1���ռ�һƿ������̼����ֹˮ��
K1
K1
��K3
K3
���ر�K2
K2
���ӷ�Һ©����װ��A�м���������ϡ���ᣬд��A�з�����Ӧ�Ļ�ѧ����ʽCaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��������D���д������Dz���ʱ��CO2�Ѽ�����д��D�з�����Ӧ�Ļ�ѧ����ʽCO2+Ca��OH��2=CaCO3��+H2O
CO2+Ca��OH��2=CaCO3��+H2O
����2��֤��������̼���������Ʒ����˻�ѧ��Ӧ���ر�ֹˮ��K1��K3����ֹˮ��K2���ӷ�Һ©����װ��C�м����������з�̪������������Һ��ֱ��ʵ���������д���˹�����C�з�����Ӧ�Ļ�ѧ����ʽ
2NaOH+CO2=Na2CO3+H2O�� Na2CO3+2HCl=2NaCl+H2O+CO2��
2NaOH+CO2=Na2CO3+H2O�� Na2CO3+2HCl=2NaCl+H2O+CO2��
��C�й۲쵽�������У���֤��CO2��NaOHȷʵ�����˻�ѧ��Ӧ����C�������ݲ���
C�������ݲ���
����������1������ͼʾ�ռ�һƿ������̼��֪��Ҫ��ֹˮ��K1��K3���ر�K2��������д��ѧ����ʽ�IJ��裺д��ע�ȣ���ȷ��д��ѧ����ʽ��
��2�����ݶ�����̼�Ļ�ѧ���ʽ�𣬸���ѹǿԭ��������������ȷ��д��ѧ����ʽ��
��2�����ݶ�����̼�Ļ�ѧ���ʽ�𣬸���ѹǿԭ��������������ȷ��д��ѧ����ʽ��
����⣺��1����ֹˮ��K1��K3���ر�K2���ӷ�Һ©����װ��A�м���������ϡ���ᣬϡ������A�д���ʯ��Ӧ���ɶ�����̼����ʱװ��A�з�����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
������D���д������Dz���ʱ��CO2�Ѽ�����D�з�����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��2���ر�K1��K3����ֹˮ��K2���ӷ�Һ©����װ��C�м����������з�̪������������Һ������ƿ�еĶ�����̼���������Ʒ�Ӧ��װ��C�з�Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2=Na2CO3+H2O��
��ƿ�еĶ�����̼���������Ʒ�Ӧ��ѹǿ��С��Bװ���е�ѹǿ�ͻὫ�ձ��ڵ���������C�У�Ȼ�������뷴Ӧ���ɵ�̼���Ʒ�Ӧ�����ɶ�����̼��������������ɣ�
�ʴ�Ϊ��
��1��K1 �� K3 �� K2 ��
CaCO3+2HCl=CaCl2+H2O+CO2����CO2+Ca��OH��2=CaCO3��+H2O��
��2��2NaOH+CO2=Na2CO3+H2O
Na2CO3+2HCl=2NaCl+H2O+CO2����
C�������ݲ�����
������D���д������Dz���ʱ��CO2�Ѽ�����D�з�����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��2���ر�K1��K3����ֹˮ��K2���ӷ�Һ©����װ��C�м����������з�̪������������Һ������ƿ�еĶ�����̼���������Ʒ�Ӧ��װ��C�з�Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2=Na2CO3+H2O��
��ƿ�еĶ�����̼���������Ʒ�Ӧ��ѹǿ��С��Bװ���е�ѹǿ�ͻὫ�ձ��ڵ���������C�У�Ȼ�������뷴Ӧ���ɵ�̼���Ʒ�Ӧ�����ɶ�����̼��������������ɣ�
�ʴ�Ϊ��
��1��K1 �� K3 �� K2 ��
CaCO3+2HCl=CaCl2+H2O+CO2����CO2+Ca��OH��2=CaCO3��+H2O��
��2��2NaOH+CO2=Na2CO3+H2O
Na2CO3+2HCl=2NaCl+H2O+CO2����
C�������ݲ�����
���������⿼���˶�����̼�����ʼ���ѧ����ʽ����д���ѵ����������ڻ�ѧ��Ӧ������װ����ѹǿ�ĸı䣮

��ϰ��ϵ�д�
�����Ŀ
 ��2012?��̨��һģ������ʵ�������еĺᡢ�������ʾ����������ͼ��ʾ�仯���Ƶ��ǣ�������
��2012?��̨��һģ������ʵ�������еĺᡢ�������ʾ����������ͼ��ʾ�仯���Ƶ��ǣ�������