��Ŀ����
��8�֣�ij��ѧ��ȤС��ͨ������ʵ���ɷ���м�Ʊ������������壨FeSO4��7H2O����
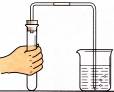
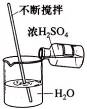
����ϴ�ӹ��ķ���м�м�������ϡ���ᣬ��Ӧ��������ˡ�
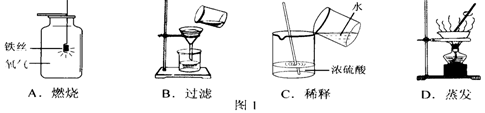
�ڽ���Һת�뵽�ܱ������У����á���ȴ���������������塣
�۴��ᾧ��Ϻ��˳����壬���������µ�ˮϴ�Ӿ���3�Ρ�
��ش��������⣺
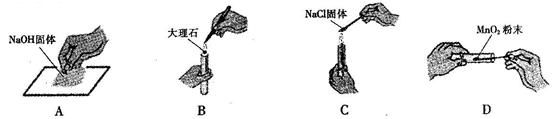
��1���ڲ���ٹ���ʱ�������õ��IJ��������У�����©�����ձ��⣬���� ��
��2��д��������з�����Ӧ�Ļ�ѧ��Ӧ����ʽ
��3���������ϴ�Ӿ����õ�ˮ���������ҵ��£���ԭ����
��4��Ϊ���жϲ�����о�����������������Ƿ�ϴ�����������3��ϴ��Һ�еμ����ᱵ��Һ��ϡ���ᣬ�õ���ɫ�������Դ�֤���þ��岢δϴ�����ý����Ƿ�ɿ���Ϊʲô��
����ϴ�ӹ��ķ���м�м�������ϡ���ᣬ��Ӧ��������ˡ�
�ڽ���Һת�뵽�ܱ������У����á���ȴ���������������塣
�۴��ᾧ��Ϻ��˳����壬���������µ�ˮϴ�Ӿ���3�Ρ�
��ش��������⣺
��1���ڲ���ٹ���ʱ�������õ��IJ��������У�����©�����ձ��⣬���� ��
��2��д��������з�����Ӧ�Ļ�ѧ��Ӧ����ʽ
��3���������ϴ�Ӿ����õ�ˮ���������ҵ��£���ԭ����
��4��Ϊ���жϲ�����о�����������������Ƿ�ϴ�����������3��ϴ��Һ�еμ����ᱵ��Һ��ϡ���ᣬ�õ���ɫ�������Դ�֤���þ��岢δϴ�����ý����Ƿ�ɿ���Ϊʲô��
��1��������
��2��Fe+H2SO4=FeSO4+H2��
��3����Ϊ����������ˮ���¶�Խ�ͣ��ܽ��ԽС����ˮԽ�٣�������ʧԽ�٣�
��4�����ɿ��������Ƿ�ϴ�ɾ���ϴ��Һ��һ������SO42-
��2��Fe+H2SO4=FeSO4+H2��
��3����Ϊ����������ˮ���¶�Խ�ͣ��ܽ��ԽС����ˮԽ�٣�������ʧԽ�٣�
��4�����ɿ��������Ƿ�ϴ�ɾ���ϴ��Һ��һ������SO42-
������˵Ļ���ʵ��������ȱ�ٲ����������������ã�������л�ѧ��ӦΪ�������ᷴӦ������ʽΪ��Fe+H2SO4=FeSO4+H2������Ϊ����������ˮ���¶�Խ�ͣ��ܽ��ԽС����ˮԽ�٣�������ʧԽ�١����ɿ��������Ƿ�ϴ�ɾ���ϴ��Һ��һ������SO42-��Ӧ�ü���H+ ��������SO42-��

��ϰ��ϵ�д�
�����Ŀ