��Ŀ����
��8�֣���ͼ��ʵ���ҳ�������ȡ�����װ�ã�

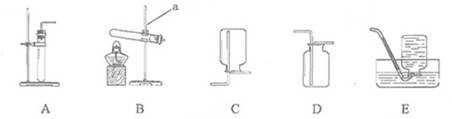
��1��д����ͼ�д�������������ƣ��� ���� ��
��2����ȡ������̼������B������װ�ã����ռ�װ��ͨ���� ������ĸ������д��ʵ�����ƶ�����̼�Ļ�ѧ����ʽ ��
��3��ʵ����������Ҫ��ȡ�������������������������Ҫ��ҩƷ�ǣ� �������ƣ���
��4����������˿��������ȼ�յ�ʵ��ʱ���Ѻ��ȵ���˿����װ�������ļ���ƿ���Ӧ���� ���ѧʽ���������ʵ��ʱʢ�����ļ���ƿԤ�ȼ�����ˮ��ˮ�������� ��

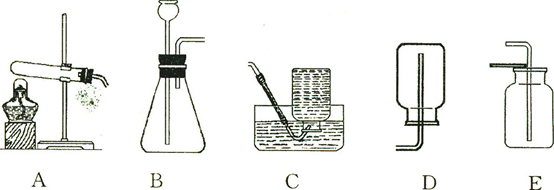
��1��д����ͼ�д�������������ƣ��� ���� ��
��2����ȡ������̼������B������װ�ã����ռ�װ��ͨ���� ������ĸ������д��ʵ�����ƶ�����̼�Ļ�ѧ����ʽ ��
��3��ʵ����������Ҫ��ȡ�������������������������Ҫ��ҩƷ�ǣ� �������ƣ���
��4����������˿��������ȼ�յ�ʵ��ʱ���Ѻ��ȵ���˿����װ�������ļ���ƿ���Ӧ���� ���ѧʽ���������ʵ��ʱʢ�����ļ���ƿԤ�ȼ�����ˮ��ˮ�������� ��
��8�֣���1���� �ƾ��ƣ�1�֣��� �� ����©�� ��1�֣�
��2��E ��1�֣���CaCO3 + 2HCl�� CaCl2+ CO2��+ H2O ��2�֣���д��������1�֣�δ��ƽҲ��1�֣����߶�ȱҲֻ��1�֣�
��3���������/����غͶ������� ��1�֣�ֻдһ��������ɵ����֣�
��4��Fe3O4 ��1�֣�����ֹ����ƿ�������ѣ�1�֣�����������Ҳ���֣�
��2��E ��1�֣���CaCO3 + 2HCl�� CaCl2+ CO2��+ H2O ��2�֣���д��������1�֣�δ��ƽҲ��1�֣����߶�ȱҲֻ��1�֣�
��3���������/����غͶ������� ��1�֣�ֻдһ��������ɵ����֣�
��4��Fe3O4 ��1�֣�����ֹ����ƿ�������ѣ�1�֣�����������Ҳ���֣�
����1����dz��õ��������ƣ��پƾ��ƣ��ڳ���©����
��2����ȡ������̼������B������װ�ã����ռ�װ��ͨ��E����ȡ������̼��ѧ����ʽΪ��CaCO3+2HCl=CaCl2+CO2��+H2O��
��3��ʵ����������Ҫ��ȡ�������������������������Ҫ��ҩƷ�ǣ�������ػ�����غͶ������̻�˫��ˮ��
��4����������˿��������ȼ�յ�ʵ��ʱ���Ѻ��ȵ���˿����װ�������ļ���ƿ���Ӧ����Fe3O4�������ʵ��ʱʢ�����ļ���ƿԤ�ȼ�����ˮ��ˮ�������ǣ���ֹ����ƿ�������ѣ�
�ʴ�Ϊ����1���پƾ��ƣ��ڳ���©����
��2��E��CaCO3+2HCl=CaCl2+CO2��+H2O��
��3��������ػ�����غͶ������̻�˫��ˮ��
��4��Fe3O4����ֹ����ƿ�������ѣ�
��2����ȡ������̼������B������װ�ã����ռ�װ��ͨ��E����ȡ������̼��ѧ����ʽΪ��CaCO3+2HCl=CaCl2+CO2��+H2O��
��3��ʵ����������Ҫ��ȡ�������������������������Ҫ��ҩƷ�ǣ�������ػ�����غͶ������̻�˫��ˮ��
��4����������˿��������ȼ�յ�ʵ��ʱ���Ѻ��ȵ���˿����װ�������ļ���ƿ���Ӧ����Fe3O4�������ʵ��ʱʢ�����ļ���ƿԤ�ȼ�����ˮ��ˮ�������ǣ���ֹ����ƿ�������ѣ�
�ʴ�Ϊ����1���پƾ��ƣ��ڳ���©����
��2��E��CaCO3+2HCl=CaCl2+CO2��+H2O��
��3��������ػ�����غͶ������̻�˫��ˮ��
��4��Fe3O4����ֹ����ƿ�������ѣ�

��ϰ��ϵ�д�
�����Ŀ