��Ŀ����
ijУ����С��ͬѧ��������ɳ��̼�������ʵ�ʳ����Ʒ����������ʵ�飺��һֻ60g���ձ��м���140g��Ʒ��Ȼ���5�μ������ᣬÿ�μ�������70g��������ˮ���Ȼ����ݳ�����ÿ�η�Ӧ��ȫ�����ձ����ձ������ʵ�����������¼ʵ���������£�| ����ϡ������� | 1 | 2 | 3 | 4 | 5 |
| �ձ����ձ������ʵ�������/g | 267.8 | 335.6 | 403.4 | 471.2 | 541.2 |
��ش��������⣺
��1����Ӧ�����в���������̼��������Ϊ______��
��2��ʳ����Ʒ����Ԫ������Ԫ�ص��������Ƕ��٣�
��3����ʵ������Һȫ��ת��Ϊ�Ȼ�����Һ������Ҫ����̼���Ʒ�ĩ�������Ƕ��٣�
��2�����ݻ�ѧ����ʽ����֪������̼�����������̼���Ƶ��������Ӷ�����Ȼ��Ƶ���������Ʒ����Ԫ�ص�����Ϊ�Ȼ�����̼��������Ԫ������֮�ͣ���Ԫ������Ϊ�Ȼ�������Ԫ��������
��3�������������ݣ�ÿ70g������̼���Ʒ�Ӧ����2.2g������̼������μ������������û�μӷ�Ӧ�����ʣ����Һ����70gϡ����û��Ӧ�����ݶ�����̼���������������̼���Ƶ�������
����⣺��1�����ݱ������������ݿ�֪���������ᵽ�����֮�����ʵ��������ڷ����仯��˵����ʱ��Ʒȫ����Ӧ�����ɵĶ�����̼����Ϊ60g+140g+350g-541.2g=8.8 g
��2����ʳ����Ʒ��̼���Ƶ�����Ϊx��
Na2CO3+2HCl�T2NaCl+CO2��+H2O
106 44
x 8.8 g
��ã�x=21.2 g
ʳ����Ʒ��NaԪ�ص�������ClԪ�ص�������=[��140g-21.2 g-1.8 g ��×
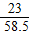 +21.2 g×
+21.2 g×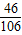 ]����140g-21.2 g
]����140g-21.2 g-1.8 g��×
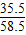 =276��355����55.2��71��
=276��355����55.2��71����ʳ����Ʒ����Ԫ������Ԫ�ص���������276��355��
��3���⣺�軹��Ҫ̼���Ʒ�ĩ������Ϊy��
Na2CO3+2HCl�T2NaCl+CO2��+H2O
106 44
y 2.2 g
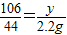
��ã�y=5.3 g
�𣺻���Ҫ̼���Ʒ�ĩ5.3 g��
�ʴ�Ϊ����1��8.8 g ��2��276��355 ��3��5.3 g
������������һ����ѧ����ʽ��ͼ��������һ��ļ����⣬�ѶȽϴ�ѧ������������������ϸߣ�

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д���2011��ɽ��Ϋ����26�⣩������������ʹ����������������������һ����Ҫ�ı�־��
��1��ÿ�������ʴ������ɾ����ʧ������Ʒ��ʴ����Ҫԭ����
��
��2��У������ȤС���ͬѧ��ȥΫ��ij������������Ʒ����ʵ�飺���ķݲ�ͬ������������Ʒ���ٶ�����ֻ���������͵���̼���ֱ�ӵ�100g����������ͬ��ϡ�����У���ַ�Ӧ��õ�ʵ���������±�����֪���ڱ�״���£�22.4LH2������Ϊ2g��
| ʵ����� | 1 | 2 | 3 | 4 |
| ������Ʒ������ / g | 2.88 | 5.76 | 9.24 | 10.28 |
| ����H2���������״���£�/L | 1.12 | 2.24 | 3.36 | m |
ͨ������ش��������⣺
������������m��ֵΪ ��
�ڸ��ݱ������ݼ���ϡ������H2SO4������������
��2011��ɽ��Ϋ����26�⣩������������ʹ����������������������һ����Ҫ�ı�־��
��1��ÿ�������ʴ������ɾ����ʧ������Ʒ��ʴ����Ҫԭ����
��
��2�� У������ȤС���ͬѧ��ȥΫ��ij������������Ʒ����ʵ�飺���ķݲ�ͬ��������
����Ʒ���ٶ�����ֻ���������͵���̼���ֱ�ӵ�100g����������ͬ��ϡ�����У���ַ�Ӧ��õ�ʵ���������±�����֪���ڱ�״���£�22.4LH2������Ϊ2g��
| ʵ����� | 1 | 2 | 3 | 4 |
| ������Ʒ������ / g | 2.88 | 5.76 | 9.24 | 10.28 |
| ����H2���������״���£�/L | 1.12 | 2.24 | 3.36 | m |
ͨ������ش��������⣺
������������m��ֵΪ ��
�ڸ��ݱ������ݼ���ϡ������H2SO4������������
��8�֣�������������ʹ����������������������һ����Ҫ�ı�־��
��1��ÿ�������ʴ������ɾ����ʧ������Ʒ��ʴ����Ҫԭ����
��
У������ȤС���ͬѧ��ȥΫ��ij������������Ʒ����ʵ�飺���ķݲ�ͬ������������Ʒ���ٶ�����ֻ���������͵���̼���ֱ�ӵ�100g����������ͬ��ϡ�����У���ַ�Ӧ��õ�ʵ���������±�����֪���ڱ�״���£�22.4LH2������Ϊ2g��
| ʵ����� | 1 | 2 | 3 | 4 |
| ������Ʒ������ / g | 2.88 | 5.76 | 9.24 | 10.28 |
| ����H2���������״���£�/L | 1.12 | 2.24 | 3.36 | m |
������������m��ֵΪ ��
�ڸ��ݱ������ݼ���ϡ������H2SO4������������

