��Ŀ����
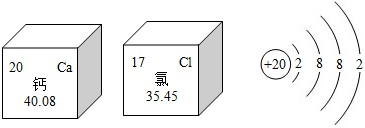 ��2013?������һģ�� Ԫ�����ڱ�����ѧϰ���о���ѧ�Ĺ��ߣ���ͼ�Ǹơ�������Ԫ�ص������Ϣ��
��2013?������һģ�� Ԫ�����ڱ�����ѧϰ���о���ѧ�Ĺ��ߣ���ͼ�Ǹơ�������Ԫ�ص������Ϣ����ش�
��1����Ԫ������
����
����
����������ǽ�������Ԫ�أ����ԭ������Ϊ40.08
40.08
����2����Ԫ������Ԫ����ʵ�������������
������
��ͬ����3����Ԫ�ص�ԭ���ڻ�ѧ�仯������
ʧȥ
ʧȥ
����õ�����ʧȥ���������γɸ����ӣ�������Ԫ���γɻ�����Ļ�ѧʽΪCaCl2
CaCl2
����4����ͯȱ�ƿ��ܻᵼ��
���Ͳ�
���Ͳ�
���ƶѪ֢�������Ͳ���������5���ø�Ԫ���Ʊ��IJ��Ʋ��������ơ�Ca��IO3��2�������е�Ԫ�صĻ��ϼ�Ϊ
+5
+5
�ۣ���������1�����ݽ���Ԫ������һ���С������ԡ����ԭ������ΪԪ����������������н��
��2������Ԫ����ָ������ͬ����������ͬ�˵������ͬһ��ԭ�ӵ��ܳƽ��н��
��3�����ݸƵ�ԭ�ӽṹʾ��ͼ���������������з���������������С��4����ʧȥ���ӣ����ݻ��ϼ���д��ѧʽ��
��4������������ȱ�ƻ�����Ͳ��ͷ������������Ծݴ˽��
��5�����ݻ������������ϼ۵Ĵ�����Ϊ�㣬���Լ����Ԫ�صĻ��ϼۣ�
��2������Ԫ����ָ������ͬ����������ͬ�˵������ͬһ��ԭ�ӵ��ܳƽ��н��
��3�����ݸƵ�ԭ�ӽṹʾ��ͼ���������������з���������������С��4����ʧȥ���ӣ����ݻ��ϼ���д��ѧʽ��
��4������������ȱ�ƻ�����Ͳ��ͷ������������Ծݴ˽��
��5�����ݻ������������ϼ۵Ĵ�����Ϊ�㣬���Լ����Ԫ�صĻ��ϼۣ�
����⣺��1������Ԫ������һ���С������ԣ���˸�Ԫ�����ڽ���Ԫ�أ���ͼ�����Ƶ����ԭ������Ϊ40.08��
�ʴ�Ϊ��������40.08��
��2��Ԫ����ָ������ͬ����������ͬ�˵������ͬһ��ԭ�ӵ��ܳƣ����Ա��в�ͬ��Ԫ����ʵ���������������ͬ��
�ʴ�Ϊ����������
��3����ԭ�ӵ�������������2��С��4�����Ի�ѧ��Ӧ����ʧ��������2�����ӣ�ʹ������Ϊ����㣬�ﵽ8�����ȶ��ṹ���Ӷ���������λ������ɣ��仯�ϼ۳�����Ϊ+2�ۣ�����Ԫ�صĻ��ϼ۳�����Ϊ-1�ۣ������γɻ�����Ļ�ѧʽΪ��CaCl2��
�ʴ�Ϊ��CaCl2��
��4������������ȱ�ƻ�����Ͳ��ͷ���������
�ʴ�Ϊ�����Ͳ���
��5�����ݻ������������ϼ۵Ĵ�����Ϊ�㣬�������Ԫ�صĻ��ϼ�Ϊx����+2+[x+��-2����3]��2=0����ã�x=+5��
�ʴ�Ϊ��+5��
�ʴ�Ϊ��������40.08��
��2��Ԫ����ָ������ͬ����������ͬ�˵������ͬһ��ԭ�ӵ��ܳƣ����Ա��в�ͬ��Ԫ����ʵ���������������ͬ��
�ʴ�Ϊ����������
��3����ԭ�ӵ�������������2��С��4�����Ի�ѧ��Ӧ����ʧ��������2�����ӣ�ʹ������Ϊ����㣬�ﵽ8�����ȶ��ṹ���Ӷ���������λ������ɣ��仯�ϼ۳�����Ϊ+2�ۣ�����Ԫ�صĻ��ϼ۳�����Ϊ-1�ۣ������γɻ�����Ļ�ѧʽΪ��CaCl2��
�ʴ�Ϊ��CaCl2��
��4������������ȱ�ƻ�����Ͳ��ͷ���������
�ʴ�Ϊ�����Ͳ���
��5�����ݻ������������ϼ۵Ĵ�����Ϊ�㣬�������Ԫ�صĻ��ϼ�Ϊx����+2+[x+��-2����3]��2=0����ã�x=+5��
�ʴ�Ϊ��+5��
�����������ۺ���ǿ���ѶȲ�����Ҫ����Ӧ��Ԫ�����ڱ����ṩ����Ϣ���з��������������Ԫ�صĸ����ԭ�ӽṹ�Լ���������Ԫ�ػ��ϼ۵ļ���ȣ�

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ
 ��2013?������һģ��������ͼ��ʾ����Һ©����ʹ���е���ɫҺ�壨���������Թ��еĹ��壨�������Ӵ���Ӧ���ɹ۲쵽�����������ʹ��������ֱ�д��һ������ͼ�����������Ҫ��Ļ�ѧ����ʽ��
��2013?������һģ��������ͼ��ʾ����Һ©����ʹ���е���ɫҺ�壨���������Թ��еĹ��壨�������Ӵ���Ӧ���ɹ۲쵽�����������ʹ��������ֱ�д��һ������ͼ�����������Ҫ��Ļ�ѧ����ʽ�� ��2013?������һģ��A��B��C��D��E ���dz��л�ѧ���������ʣ�����AΪ���Σ�B��D��Ϊ����������Ԫ�ص����壬X��Y��Ϊ��ɫ��ĩ��FΪ��ɫ�������ʣ�E�ڳ�����ΪҺ̬������֮���ת����ϵ����ͼ��ʾ����Ӧ�����Ͳ��ֲ�������ȥ�����Իش��������⣮
��2013?������һģ��A��B��C��D��E ���dz��л�ѧ���������ʣ�����AΪ���Σ�B��D��Ϊ����������Ԫ�ص����壬X��Y��Ϊ��ɫ��ĩ��FΪ��ɫ�������ʣ�E�ڳ�����ΪҺ̬������֮���ת����ϵ����ͼ��ʾ����Ӧ�����Ͳ��ֲ�������ȥ�����Իش��������⣮