��Ŀ����
����һ�ֲ���������ͭ��ĩ��Ʒ������Ϊͭ�ۣ���С�պ�Сǿͨ��ʵ��ⶨ��Ʒ������ͭ����������������ʾ��CuO+CO Cu+CO2������ش�������⣺
Cu+CO2������ش�������⣺
��1��С�ճ�ȡ10g��Ʒ��������ͼ��ʾװ�ý���ʵ�飬��ַ�Ӧ��Bװ������4.4g���ݴ˼�������ͭ��Ʒ������ͭ������������
�⣺________
��________
��2��Сǿͬѧ��ͬ����װ�ý���ʵ�飬ʵ������С�����õ���ȷ����Ƚϣ�����ƫ��������������Ŀ���ԭ��________��
��3��С�������û�ԭ����ͭ��ԭ�����вⶨ�������Ҫд�������ⶨ��Ʒ������ͭ�����������ķ�����Ҫ�ⶨ�����ݣ�������������������д������������ֵ��________��
�⣺��1��Bװ������4.4g��Ϊ��Ӧ���ɵĶ�����̼����������μ���Ӧ����ͭ������Ϊx��
CuO+CO Cu+CO2
Cu+CO2
80 44
x 4.4g
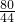 =
=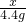
���x=8.0g
����Ʒ������ͭ����������=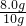 ��100%=80%
��100%=80%
�𣺸���Ʒ������ͭ����������80%��
��2��������ֹͣͨ������ʹ����ͭû�б���ȫ��ԭ����ʹ����������Һ�������Ӽ�С����ɲ�ý��ƫС��
�ʴ�Ϊ��������ֹͣͨ������ʹ����ͭû�б���ȫ��ԭ��
��3���Ȳ������ͭ��Ʒ���������ٲ������ͭ��Ʒ�Ͳ����ܵ�������Ȼ��ͨһ����̼����ʹ�������ͭ��ȫ��Ӧ��ʵ�����������Ͳ����ܵ������������������ٵIJ���������ͭ����Ԫ�ص����������������ͭ���������ٸ�������������ʽ������Ʒ������ͭ������������
�ʴ�Ϊ���Ȳ������ͭ��Ʒ���������ٲ������ͭ��Ʒ�Ͳ����ܵ�������Ȼ��ͨһ����̼����ʹ�������ͭ��ȫ��Ӧ��ʵ�����������Ͳ����ܵ������������������ٵIJ���������ͭ����Ԫ�ص����������������ͭ���������ٸ�������������ʽ������Ʒ������ͭ������������
��������1������Bװ������4.4g��Ϊ��Ӧ���ɵĶ�����̼��������ϸ��ݻ�ѧ����ʽ�ļ��㲽����㼴�ɣ�
��2������������ԭ����ͭ��ע�����������
��3���Ȳ������ͭ��Ʒ���������ٲ������ͭ��Ʒ�Ͳ����ܵ�������Ȼ��ͨһ����̼����ʹ�������ͭ��ȫ��Ӧ��ʵ�����������Ͳ����ܵ������������������ٵIJ���������ͭ����Ԫ�ص����������������ͭ���������ٸ�������������ʽ������Ʒ������ͭ������������
������������Ҫ����һ����̼����ԭ����ͭʱ��ע�������Լ����ݻ�ѧ����ʽ�ļ��㡢ʵ�鷽������Ƶ�֪ʶ���ѶȽϴ�
CuO+CO
 Cu+CO2
Cu+CO280 44
x 4.4g
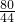 =
=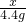
���x=8.0g
����Ʒ������ͭ����������=
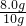 ��100%=80%
��100%=80%�𣺸���Ʒ������ͭ����������80%��
��2��������ֹͣͨ������ʹ����ͭû�б���ȫ��ԭ����ʹ����������Һ�������Ӽ�С����ɲ�ý��ƫС��
�ʴ�Ϊ��������ֹͣͨ������ʹ����ͭû�б���ȫ��ԭ��
��3���Ȳ������ͭ��Ʒ���������ٲ������ͭ��Ʒ�Ͳ����ܵ�������Ȼ��ͨһ����̼����ʹ�������ͭ��ȫ��Ӧ��ʵ�����������Ͳ����ܵ������������������ٵIJ���������ͭ����Ԫ�ص����������������ͭ���������ٸ�������������ʽ������Ʒ������ͭ������������
�ʴ�Ϊ���Ȳ������ͭ��Ʒ���������ٲ������ͭ��Ʒ�Ͳ����ܵ�������Ȼ��ͨһ����̼����ʹ�������ͭ��ȫ��Ӧ��ʵ�����������Ͳ����ܵ������������������ٵIJ���������ͭ����Ԫ�ص����������������ͭ���������ٸ�������������ʽ������Ʒ������ͭ������������
��������1������Bװ������4.4g��Ϊ��Ӧ���ɵĶ�����̼��������ϸ��ݻ�ѧ����ʽ�ļ��㲽����㼴�ɣ�
��2������������ԭ����ͭ��ע�����������
��3���Ȳ������ͭ��Ʒ���������ٲ������ͭ��Ʒ�Ͳ����ܵ�������Ȼ��ͨһ����̼����ʹ�������ͭ��ȫ��Ӧ��ʵ�����������Ͳ����ܵ������������������ٵIJ���������ͭ����Ԫ�ص����������������ͭ���������ٸ�������������ʽ������Ʒ������ͭ������������
������������Ҫ����һ����̼����ԭ����ͭʱ��ע�������Լ����ݻ�ѧ����ʽ�ļ��㡢ʵ�鷽������Ƶ�֪ʶ���ѶȽϴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

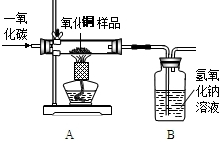
 Cu+CO2������ش�������⣺
Cu+CO2������ش�������⣺