��Ŀ����
��6�֣���Ԫ������������Ԫ�ء���������������Ԫ��֮һ���������������ȱ������������ͼ��ij�������IJ��ֱ�ǩ��������ѧ֪ʶ�ش�����:
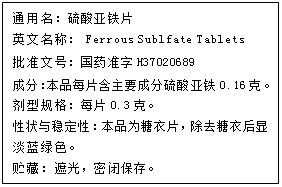
��1������ȱ�������� ��
��2��ʵ����Ҫ�÷���м��ϡ���ᷴӦ��ȡ����������
�ٷ�ӦǰҪ��ȥ����м��������ۣ�ϴ���������õ���Һ�� �������ţ�
�ڷ�Ӧ�Ļ�ѧ����ʽ�� ����Ӧǰ�ϼ۷����仯��Ԫ���� ��дԪ�ط��ţ���
��3�����ݱ�ǩ�е���Ϣ���㣬����һƬ��ҩ�൱�ڲ��� g������2λС������

��1������ȱ�������� ��
��2��ʵ����Ҫ�÷���м��ϡ���ᷴӦ��ȡ����������
�ٷ�ӦǰҪ��ȥ����м��������ۣ�ϴ���������õ���Һ�� �������ţ�
| A��̼������Һ | B��ϡ���� |
| C������������Һ | D��ʯ��ˮ |
��3�����ݱ�ǩ�е���Ϣ���㣬����һƬ��ҩ�൱�ڲ��� g������2λС������
��1��ƶѪ ��2����C �� Fe + H2SO4 = FeSO4 + H2����Fe��H ��3��0.06
��1������Ѫ�쵰���к�����Ԫ�أ�ȱ���ᵼ������ƶѪ��
��2��ϴ���������õ���Һ������������Һ����Ӧ�Ļ�ѧ����ʽ��Fe + H2SO4 = FeSO4 + H2������Ӧǰ�ϼ۷����仯��Ԫ����Fe��H
��3������������������Ԫ�ص��������������н��
��2��ϴ���������õ���Һ������������Һ����Ӧ�Ļ�ѧ����ʽ��Fe + H2SO4 = FeSO4 + H2������Ӧǰ�ϼ۷����仯��Ԫ����Fe��H
��3������������������Ԫ�ص��������������н��

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ



