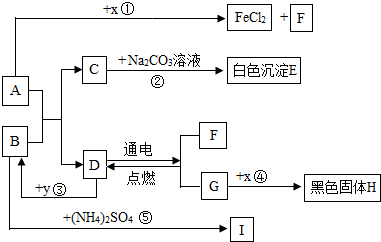
��Ŀ����
��ѧ���ʼ���仯������������ء�
�� �����˵���ҺpH����ά����һ����Χ�ڡ������ҺpH��Խ������Χ���ͻᵼ��ijЩ�����IJ�����θҺ��pH 7��ѡ���������������=�����������ڵĶ�����̼�ų�����ʱ���ᵼ��ѪҺpH ��ѡ����ߡ����͡�����
�� ����ˮ�к���������Ca(HCO3)2��MgSO4�ȿ������Ρ��տ�ˮ�ĺ��л����ˮ����ԭ��֮һ�������е�Ca(HCO3)2�����˷ֽⷴӦ�����������ܵ�CaCO3����д��Ca(HCO3)2���ȷֽ�Ļ�ѧ����ʽ�� ��ʵ������������ˮ����Ba(OH)2��Һ�����ܳ��ֵ������� ��������������ԭ���ǣ����û�ѧ����ʽ��ʾ���� _____ ��
�� �����˵���ҺpH����ά����һ����Χ�ڡ������ҺpH��Խ������Χ���ͻᵼ��ijЩ�����IJ�����θҺ��pH 7��ѡ���������������=�����������ڵĶ�����̼�ų�����ʱ���ᵼ��ѪҺpH ��ѡ����ߡ����͡�����
�� ����ˮ�к���������Ca(HCO3)2��MgSO4�ȿ������Ρ��տ�ˮ�ĺ��л����ˮ����ԭ��֮һ�������е�Ca(HCO3)2�����˷ֽⷴӦ�����������ܵ�CaCO3����д��Ca(HCO3)2���ȷֽ�Ļ�ѧ����ʽ�� ��ʵ������������ˮ����Ba(OH)2��Һ�����ܳ��ֵ������� ��������������ԭ���ǣ����û�ѧ����ʽ��ʾ���� _____ ��
(1) ��������
(2) Ca(HCO3)2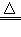 CaCO3��+H2O +CO2������ɫ������Ba(OH)2 + MgSO4== BaSO4��+Mg(OH)2��
CaCO3��+H2O +CO2������ɫ������Ba(OH)2 + MgSO4== BaSO4��+Mg(OH)2��
(2) Ca(HCO3)2
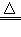 CaCO3��+H2O +CO2������ɫ������Ba(OH)2 + MgSO4== BaSO4��+Mg(OH)2��
CaCO3��+H2O +CO2������ɫ������Ba(OH)2 + MgSO4== BaSO4��+Mg(OH)2�� 
��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ