��Ŀ����
���⣨H2S����һ����ɫ���г�����ζ�����壬�о綾���ܶȱȿ�����������ˮ��ʵ��������������FeS����ϡ���ᷴ��H2SO4����Ӧ����H2S�����FeSO4���Իش�
��1��������������� ��
��2��ʵ������ȡ��������Ļ�ѧ����ʽΪ ��
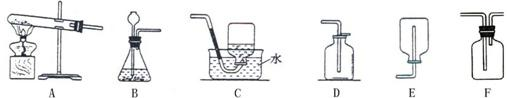
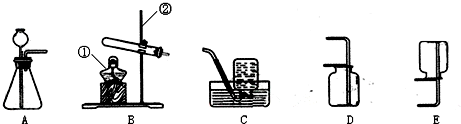
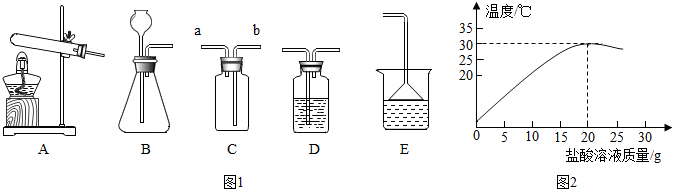
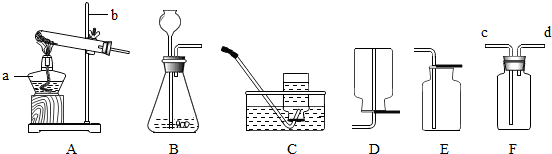
��3��ʵ������ȡ��������Ӧѡ�� ����̹̼��ȡ���Һ�����ȡ����������װ�ã��ռ����������� ����
��4�������������ȼ�գ�������������ʱ��ȼ�յIJ����ǵ������ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ
��5������������ʱ��ȼ�յIJ����Ƕ��������ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ
��1��������������� ��
��2��ʵ������ȡ��������Ļ�ѧ����ʽΪ ��
��3��ʵ������ȡ��������Ӧѡ�� ����̹̼��ȡ���Һ�����ȡ����������װ�ã��ռ����������� ����
��4�������������ȼ�գ�������������ʱ��ȼ�յIJ����ǵ������ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ
��5������������ʱ��ȼ�յIJ����Ƕ��������ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ
��1����ɫ���г�����ζ�����塢�ܶȱȿ�����������ˮ��
��2��FeS + H2SO4== FeSO4+H2S����
��3����Һ�����ȣ������ſ�������
��4��2H2S+O2 2H2O + 2S��
2H2O + 2S��
��5��2H2S + 3O2 2H2O + 2SO2
2H2O + 2SO2
��2��FeS + H2SO4== FeSO4+H2S����
��3����Һ�����ȣ������ſ�������
��4��2H2S+O2
 2H2O + 2S��
2H2O + 2S�� ��5��2H2S + 3O2
 2H2O + 2SO2
2H2O + 2SO2 
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ