��Ŀ����
����Ŀ��������ij��ѧ��ѧ��ȤС����������װ���Ʊ�����������֤���������ʣ���Ҫ��ش��������⣺
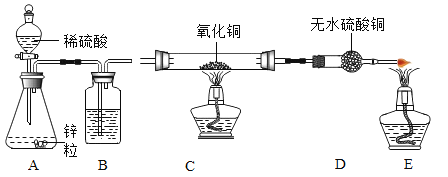
(1)��ѧʵ����������������Ϊ98%��Ũ���ᣬ������245g��������10%��ϡ���ᣬ���Ʋ������£�
�ټ��㣺������������98%��Ũ����______g������ȡ����ϡ����ȡ��ϡ��Ũ�������õIJ���������______________����Ҫ����Ũ�����ϡ�ͷ���___________��
(2)����ȤС���Ʊ�������ԭ��Ϊ___________(��ѧ����ʽ)��
(3)װ��B������__________________��
(4)�����й�ʵ�������������������ȷ������____��
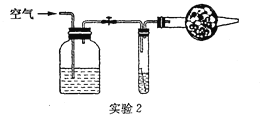
A��ʵ�鿪ʼʱ��Ӧ��ͨ��������Ȼ���ٵ�ȼC���ƾ���
B����ȼC���ƾ��ƺ�D������ͭ�����ɰ�ɫ��Ϊ��ɫ
C��E���ƾ��Ƶ������ǵ�ȼδ��Ӧ������
D��ʵ�����ʱ��Ӧ��ֹͣͨ��������Ȼ��Ϩ��C���ƾ���
(5)ʵ��������ֲ�������ͭδ����ԭ����֪��Ӧǰ����ͭ����Ϊa�ˣ���Ӧ�������Ϲ�������Ϊb�ˣ���������ԭ������ͭ������Ϊ___________g��
���𰸡� 25 ��Ͳ���ձ��������� ��Ũ�������ձ��ڻ�����ע��ˮ�У��ò��������Ͻ��� Zn+H2SO4=ZnSO4+H2�� ���������е�ˮ���� D 5(a-b)
�������� (1)��������245g��������10%��ϡ���ᣬ��Ҫ��������Ϊ98%��Ũ���ᣬ������Ϊx����245g��10%= 98%��x��x=25g����ȡҺ��ʱӦ���㵹���ӽ��̶��ߣ�Ȼ���ý�ͷ�ιܵμ����̶��ߣ������õ�����������Ͳ�ͽ�ͷ�ι���ϡ��Ũ���ᣬӦ��Ũ��������������ע��ˮ�У��������ò��������裬ʹ������ʱ��ɢ��(2)п��ϡ���ᷴӦ��������п����������Ӧ����ʽΪ��Zn+H2SO4=ZnSO4+H2����(3)�˷�Ӧ�IJ�������ˮ��Ϊ�˲�Ӱ�����ļ��飬�ڷ�ӦǰҪ���ջ�������е�ˮ��(4)A��ʵ�鿪ʼʱ��Ӧ��ͨ��������Ȼ���ٵ�ȼC���ƾ��ƣ���ֹ���ȵ������Ϳ����Ļ�����������ը��B����ȼC���ƾ��ƺ�C������ͭ��������Ӧ����ͭ��ˮ������D������ͭ�����ɰ�ɫ��Ϊ��ɫ��C��E���ƾ��Ƶ������ǵ�ȼδ��Ӧ����������ȷ��D��ʵ�����ʱ��Ӧ��Ϩ��C���ƾ��ƣ���ȴ�����º�ֹͣͨ����������ֹ���ɵ�Ŷͭ�ٱ�������(5)��֪��Ӧǰ����ͭ����Ϊa�ˣ���Ӧ�������Ϲ�������Ϊb�ˣ����������������Ϊ(a-b),�ӷ�Ӧʵ�ʿ�֪���ٹ�����������ڲμӷ�Ӧ������ͭ��ͭԪ�ص���������������ijԪ�ص���������=![]() ����������ԭ������ͭ������Ϊ(a-b)��
����������ԭ������ͭ������Ϊ(a-b)��![]() =5(a-b)��
=5(a-b)��