题目内容
李华同学用托盘天平称取纯净的氢氧化钠于洁净烧杯中,再用量筒量取自来水倒入烧杯中,搅拌后发现溶液中有白色浑浊物。对此现象感到疑惑,于是进行了如下探究。
[提出问题]白色浑浊物是什么物质?
[查阅资料]①自来水是硬水,常常含有Ca(HCO3)2和Mg(HCO3)2。
②Ca(HCO3)2与足量的氢氧化钠反应方程式: ;Mg(HCO3)2与足量的氢氧化钠反应方程式:
;Mg(HCO3)2与足量的氢氧化钠反应方程式: 
③Mg(OH)2在热水中溶解度增大,可形成稀碱溶液。
[作出猜想]
猜想一:白色浑浊物是CaCO3
猜想二: 白色浑浊物是Mg(OH)2
猜想三:白色浑浊物是_____________。
[实验探究]
①取氢氧化钠于烧杯中,加自来水搅拌,杯壁发烫,原因是____________。 静置冷却、过滤。
②取①中过滤所得滤渣加稀盐酸,有气泡冒出,反应的化学方程式是____________。
③另取①中过滤所得滤渣和蒸馏水加热,在上层清液中再加____________试液,液体变红。
[得出结论]猜想_____________正确。
[拓展延伸]李华认为[实验探究]①过滤后的滤液是纯净的氢氧化钠溶液,对吗?你的证明方法是取少量滤液于试管中,向其中滴加CaCl2溶液,若_________________,则不是纯净的氢氧化钠溶液。
练习册系列答案
 小学课时特训系列答案
小学课时特训系列答案
相关题目
下列除去杂质的方法中,合理的是( )
选项 | 物质 | 杂质 | 除杂方法 |
A | CO | CO2 | 通入足量的NaOH溶液后,干燥 |
B | NaCl溶液 | NaOH | 加入适量稀硫酸 |
C | 铁粉 | 铜粉 | 加入足量稀硫酸,过滤 |
D | CaO | CaCO3 | 加入适量稀盐酸,过滤 |
A.A B.B C.C D.D


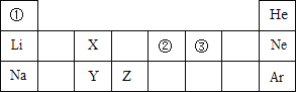
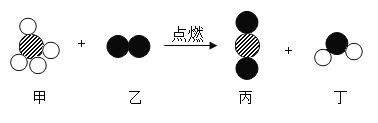


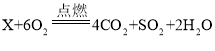 ,则噻吩X的化学式为( )
,则噻吩X的化学式为( )