��Ŀ����
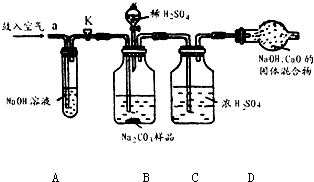
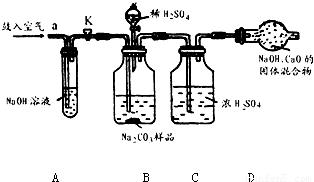
 ijNa2CO3��Ʒ�л���һЩNaCl��������װ�òⶨ�䴿�ȣ�
ijNa2CO3��Ʒ�л���һЩNaCl��������װ�òⶨ�䴿�ȣ�ʵ������������£���ȷ������10gNa2CO3��Ʒװ��Bƿ��Bװ�÷ֱ����Թ�A��Cƿ����������K����a������������������Ӻ�����K�رգ��ٽ��ѳ������ĸ����D��ƿC���ӣ�Ȼ������ϡH2SO4����ע��Bƿ�У�����Ӧ��ȫ���ٴδ���K����������������ӣ������������D������3.3g��
�ش�
��1��Aƿ��������
���տ����е�CO2
���տ����е�CO2
����2��Bƿ�з�Ӧ�Ļ�ѧ����ʽ��
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
����3�������D���ӵ���
CO2
CO2
��д��ѧʽ�����������ɴ˿��������Ʒ��Na2CO3������������79.5%
79.5%
����4����Ӧǰ��һ�ι��������������
����B��Cװ���еĿ���
����B��Cװ���еĿ���
����Ӧ���ٴι��������������ʹB��Cװ���е�CO2����ȫ������D
ʹB��Cװ���е�CO2����ȫ������D
������Ӧ����������Բⶨ�����Ӱ����ƫС
ƫС
�����ƫ��ƫС����������������Ŀ��������Ϣ��֪��Aƿ�������dz�ȥ�����еĶ�����̼��װ��B��̼���ƺ����ᷴӦ���������ƺ�ˮ�Ͷ�����̼��װ��C��������ã�װ��D���ӵ��������Ƕ�����̼�����������ݻ�ѧ����ʽ�ļ��������̼���Ƶ�������������Ӧǰ��һ�ι�������������ǣ���ȥ����װ���ڵĿ�������Ӧ���ٴι�������������ǣ�ʹB��Cװ���е�CO2����ȫ������D������Ӧ����������Բⶨ�����Ӱ����ƫС��
����⣺��1��Aƿ�������ƿ����������̼��Ӧ����Aƿ�������dz�ȥ�����еĶ�����̼���ʴ�Ϊ�����տ����е�CO2
��2��̼���ƺ����ᷴӦ���������ƺ�ˮ�Ͷ�����̼����ƽ���ɣ��ʴ�Ϊ��Na2CO3+H2SO4=Na2SO4+CO2��+H2O
��3��װ��D���ӵ��������Ƕ�����̼������������Ʒ��Na2CO3������������x
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
10gx 3.3g
=
x=79.5%
�ʴ�Ϊ��CO2 79.5%
��4����Ӧǰ��һ�ι�������������ǣ���ȥ����װ���ڵĿ�������Ӧ���ٴι�������������ǣ�ʹB��Cװ���е�CO2����ȫ������D������Ӧ����������Բⶨ�����Ӱ����ƫС���ʴ�Ϊ������B��Cװ���еĿ�����ʹB��Cװ���е�CO2����ȫ������D��ƫС
��2��̼���ƺ����ᷴӦ���������ƺ�ˮ�Ͷ�����̼����ƽ���ɣ��ʴ�Ϊ��Na2CO3+H2SO4=Na2SO4+CO2��+H2O
��3��װ��D���ӵ��������Ƕ�����̼������������Ʒ��Na2CO3������������x
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
10gx 3.3g
| 106 |
| 10gx |
| 44 |
| 3.3g |
�ʴ�Ϊ��CO2 79.5%
��4����Ӧǰ��һ�ι�������������ǣ���ȥ����װ���ڵĿ�������Ӧ���ٴι�������������ǣ�ʹB��Cװ���е�CO2����ȫ������D������Ӧ����������Բⶨ�����Ӱ����ƫС���ʴ�Ϊ������B��Cװ���еĿ�����ʹB��Cװ���е�CO2����ȫ������D��ƫС
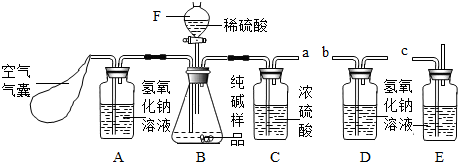
����������������ʵ��̽���⣬����ʵ����̵�̽�������л�ѧ����ʽ����д���йصļ��㣬�ۺ��ԱȽ�ǿ�����������Ŀ�ṩ����Ϣ�����ʵ��̽����һ�㲽���ѧ����֪ʶ���������Ҫע�⻯ѧ����ʽ����д����ƽ����������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
�����Ŀ
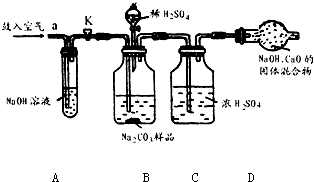 ijNa2CO3��Ʒ�л���һЩNaCl��������װ�òⶨ�䴿�ȣ�
ijNa2CO3��Ʒ�л���һЩNaCl��������װ�òⶨ�䴿�ȣ�