��Ŀ����
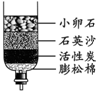 ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ��������1����Щ����ȡ���ǵĿ�ˮ��������ˮ����ͬѧ������ѧ��֪ʶ�����ǵĿ�ˮ����ͼ��ʾ�ļ���ˮ�����о���������С��ʯ��ʯӢɳ��������
����
����
����2������ط��Ըɱ��������ʵʩ������˹����꣮�ɱ������ԭ����
�ɱ��������ȣ������е�ˮ����Ѹ���������ˮ��
�ɱ��������ȣ������е�ˮ����Ѹ���������ˮ��
����3����������֮������������� ����һ���������ɣ�
��Լ��ˮ
��Լ��ˮ
����������1��С��ʯ��ʯӢɰ���˹��˵����ã������˿��Գ�ȥ������ˮ�����ʣ����Ծݴ˽����⣻
��2��Һ��������ɱ�����ʱ�ܹ��������������Ծݴ˽��
��3����Լ��ˮ�����츣��������Ծݴ˽�𣮣�
��2��Һ��������ɱ�����ʱ�ܹ��������������Ծݴ˽��
��3����Լ��ˮ�����츣��������Ծݴ˽�𣮣�
����⣺��1��С��ʯ��ʯӢɳ�ܹ����˳�������ˮ�Ĺ������ʣ������������ǹ��ˣ�
��2��Һ����ɱ������ԭ���ǣ��������Ʋ��б����̬ʱ���մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
��3����Լ����õ�����ˮ�Ĵ�ʩ��
�ʴ�Ϊ����1�����ˣ�
��2���ɱ��������ȣ������е�ˮ����Ѹ���������ˮ�Σ�
��3����Լ��ˮ��
��2��Һ����ɱ������ԭ���ǣ��������Ʋ��б����̬ʱ���մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
��3����Լ����õ�����ˮ�Ĵ�ʩ��
�ʴ�Ϊ����1�����ˣ�
��2���ɱ��������ȣ������е�ˮ����Ѹ���������ˮ�Σ�
��3����Լ��ˮ��
�����������Ҫ������⾻��ˮ�����֪ʶ��ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ


 47��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
47��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������ ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������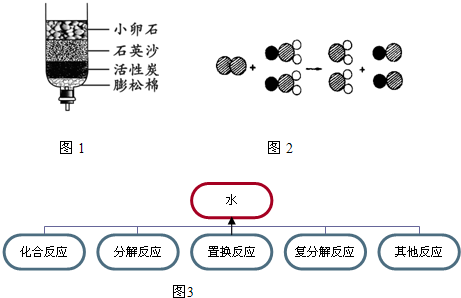
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�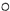 ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ