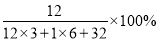��Ŀ����
�����������У���ᷢ�֡���ѧ�������ߡ���
��1��ϴ�ྫ��������ۣ�������������_____���á�
��2���Ϻ������ڰ�װ�ġ�ֱ��ˮ���������á�����̿+���˲�+�����ߡ���ˮ���ա�����̿�ڴ���_____���ã��������ˮ����_____��ѡ��������������
��3����ѧΪ��������ѩ����̿�����ҽ������������ء���ٵ���Ŀ���˶�Ա�ڱ���ǰ���ð�ɫ�ġ�þ�ۡ����֣�������Ϊ��þ�ۡ����ᡢ��ˮ�Ժã���������������þ�ۡ�����Ч�ɷ��Ǽ�ʽ̼��þ��������ȼ��300�漴�ֽ⣬��ֽ�Ļ�ѧ����ʽ�ǣ�Mg5(OH)2(CO3)4 5MgO+X+4CO2��,��X�Ļ�ѧʽ��_____��������Щ��Ϣ�������ƶϳ���þ�ۡ�����һ����;��_____��
5MgO+X+4CO2��,��X�Ļ�ѧʽ��_____��������Щ��Ϣ�������ƶϳ���þ�ۡ�����һ����;��_____��
��ϰ��ϵ�д�
�����Ŀ
��ȥ���������л��е��������ʣ���ѡ�õ��Լ���������������ȷ���ǣ� ��
��� | ���� | ���ʣ������� | �Լ��Ͳ������� |
A | O2 | H2O���� | ͨ��ŨH2SO4 |
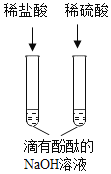
B | ϡ���� | ϡ���� | ��������BaCl2��Һ������ |
C | Na2CO3��ĩ | NaHCO3��ĩ | ���� |
D | KNO3��Һ | Ba��NO3��2��Һ | ����������Na2SO4��Һ������ |
A.A B.B C.C D.D


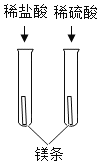 B.
B. 
 D.
D.