��Ŀ����
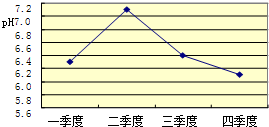
 ��2006?������һģ��ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ�����ϲ���Һ40mL���ձ��У���ε���Na2CO3��Һ����������¼����Na2CO3��Һ������x�����й����ı仯��ϵ��ͼ��ʾ��ͼ�������꣨y����ʾ
��2006?������һģ��ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ�����ϲ���Һ40mL���ձ��У���ε���Na2CO3��Һ����������¼����Na2CO3��Һ������x�����й����ı仯��ϵ��ͼ��ʾ��ͼ�������꣨y����ʾ����̼��Ƴ�����������
����̼��Ƴ�����������
��OA�η�����Ӧ�Ļ�ѧ����ʽ��Na2CO3+2HCl=2NaCl+H2O+CO2����
Na2CO3+2HCl=2NaCl+H2O+CO2����
����Ӧ���е�B��ʱ����Һ�е�������NaCl
NaCl
��C����Һ��pH��
��
�����������=��������7���������������Ĺؼ����ڣ�һ��Ū��ʵ��������������Ļ�ѧ��Ӧ������ͼ����OA��AB��BC�ε����壮
����⣺��Ϊ��Һ����Ҫ�����������Ȼ��ƣ���ε���̼������Һ��̼����Ӧ�Ⱥ����ᷴӦ����Ϊ����̼�����Ⱥ��Ȼ��Ʒ�Ӧ����̼��Ƴ�������������ڵ�����£�������̼��Ʒ�Ӧ�������Ȼ��ƣ���ʵ����̼����Ӧ�Ⱥ����ᷴӦ�����ȷ�Һ�����ᱻ̼���Ʒ�Ӧ�������ˣ�̼���ƿ�ʼ���Ȼ��Ʒ�Ӧ���ܲ���̼��Ƴ���������OA�β����г����������ķ�Ӧ��̼���ƺ�����ķ�Ӧ����ѧ����ʽ�ǣ�Na2CO3+2HCl=2NaCl+H2O+CO2���������Ȼ��Ʊ�̼���Ʒ�Ӧ��ʱ�����ﵽ���ֵ����Ӧ���е�B��ʱ��̼���ƺ��Ȼ���ǡ����ȫ��Ӧ������̼��Ƴ������Ȼ��ƣ������Һ������������NaCl�������μ�̼������Һ�������������ӣ���Һ����Ϊ�ж����̼���ƴ��ڶ��Լ��ԣ����C����Һ��pH��7���ۺϷ���ͼ�������꣨y�����ʾӦ��������̼��Ƴ�����������
�ʴ�Ϊ������̼��Ƴ�����������Na2CO3+2HCl=2NaCl+H2O+CO2����NaCl������
�ʴ�Ϊ������̼��Ƴ�����������Na2CO3+2HCl=2NaCl+H2O+CO2����NaCl������
������ѧ��ƽʱҪ��ǿ����ʵ��̽�������ϵ������Լ��ķ������⡢�������⡢������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
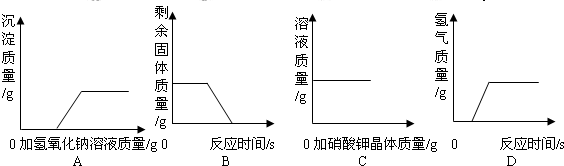
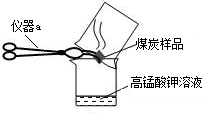
 ����Ȼ����ѧ����ʽ�����һ�����ʵĻ�ѧʽӡˢ������������������˽��������һ���ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ��
����Ȼ����ѧ����ʽ�����һ�����ʵĻ�ѧʽӡˢ������������������˽��������һ���ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ��