��Ŀ����
2008��9�£��й�������¹Ӥ���̷�����Ⱦ�¼�������ʳ��������Ⱦ�̷۵�Ӥ����������ʯ��֢����ԭ��Ҳ���̷��к��������谷�������谷��ѧʽΪC3H6N6�����ڻ��������������谷���ܶ�������ײ���Ӱ�죬���²�����ʯ�������й������谷��˵���в���ȷ����( )
A�������谷��C��H��Nԭ�ӵĸ�����Ϊ1��2��2
B�������谷�����л��ϻ���
C�������谷��3��̼ԭ�ӡ�6����ԭ�ӡ�6����ԭ�ӹ���
D�������谷�е�����������Ϊ66.7%
C
����������������������谷�Ļ�ѧʽ��C3H6N6����֪��A�������谷��C��H��Nԭ�ӵĸ�����Ϊ3��6��6=1��2��2��������ȷ��B�������谷��C��H��N����Ԫ�أ������л��ϻ��������ȷ��C��ÿ�������谷������3��̼ԭ�ӡ�6����ԭ�ӡ�6����ԭ�ӹ��ɡ��������D�������谷�е�����������Ϊ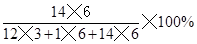 ��66.7%��������ȷ��
��66.7%��������ȷ��
���㣺���ݻ�ѧʽ�ļ���

���л�ѧ����ʽ��ȷ���ǣ�������
| A��3Fe+2AlCl3=3FeCl3+2Al | B��3Cu+2AlCl3=3CuCl2+2Al |
| C��Fe+CuCl2=FeCl2+Cu | D��2Fe+3H2SO4=Fe2��SO4��3+3H2�� |
���ܱ��������мס��ҡ��������������ʣ���һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ�����������ͼ��ʾ������˵����ȷ���ǣ�������
| A���������ǵ��� |
| B���ڸ÷�Ӧ�ж�һ��û�вμӻ�ѧ��Ӧ |
| C���÷�Ӧ�ǻ��Ϸ�Ӧ |
| D�����ҵ�����֮��һ���������ɱ������� |
�������ܱ������У����п�����ȼ�գ��������йص�����ʱ��仯��ͼ����ȷ����
A�� | B�� | C�� | D�� |
����Ԫ��������� �� ��
| A�������� | B�������� | C�������� | D����������� |
ij��ɫ��ԴX����ȼ�յĻ�ѧ����ʽ��X��3O2 2CO2��3H2O�����������غ㶨���ж�X�Ļ�ѧʽΪ�� ��
2CO2��3H2O�����������غ㶨���ж�X�Ļ�ѧʽΪ�� ��
| A��H2 | B��CH4 | C��CH3OH | D��C2H5OH |
Һ��������������������ܺĵ��ŵ�Խ��Խ�ܵ�����������������ѧ���о����֣�����Һ�����ӹ�����ʹ�õ�����������NF3��������ЧӦ�Ƕ�����̼��1.7����NF3�з�Ԫ��Ϊ-1�ۣ���Ԫ�صĻ��ϼ�Ϊ
| A��+3 | B��-3 | C��+1 | D��0 |
�ճ������г����˼ӵ�ʳ�Ρ��߸�ţ�̵ȣ�����ĵ⡢��ָ����
| A������ | B��ԭ�� | C������ | D��Ԫ�� |
