��Ŀ����
ij������������������Ϊ20%������������Һϴ��һ����ʯ�Ͳ�Ʒ�еIJ������ᡣ
��1��������������Ϊ20%������������Һ50g����Ҫ������������ g�� ˮ mL����ˮ���ܶ�Ϊ1g/cm3��
��2��ϴ��һ����ʯ�Ͳ�Ʒ�еIJ������ᣬ��������������Ϊ20%������������Һ40g,ϴ�Ӻ���Һ�����ԣ�������һ������ʯ�Ͳ�Ʒ��H2SO4������������Ӧ�Ļ�ѧ����ʽ�ǣ�2NaOH+ H2SO4 Na2SO4+2H2O ��
.��1�� 10 40
��2��
�⣺��ʯ�Ͳ�Ʒ��H2SO4������Ϊx
2NaOH + H2SO4===Na2SO4+2H2O
|
40g��20% x
|
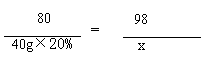
|
x=9.8g
��ʯ�Ͳ�Ʒ��H2SO4������Ϊ9.8 g��

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�
�����Ŀ
 m3��
m3��