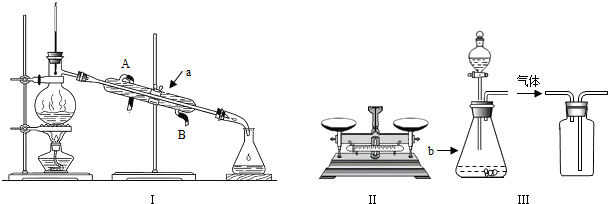
��Ŀ����
��ʵ������ȡCO2��Ӧ����Һ�����̽��
(1)��״ʯ��ʯ������ϡ���ᷴӦ�������ݳ������Ժ���,�����Һ��pH=2������Һ�� ___________�ԣ���Һ����������Ҫ������________________��
(2)�����ʵ����Ҫ����Ҫ������������Ϊ26.5%��̼������Һ200g
�ټ��㣺��Ҫ̼���ƹ��������Ϊ___________g��ˮ�����Ϊ__________mL��ˮ���ܶȽ��ƿ���1g/cm3��
�ڳ���������������ƽƽ���__________����������ƽ�����̣������������� Ȼ������������__________������̼���ƹ��壬ֱ����ƽƽ�⡣
���ܽ⣺����Ͳ��ȡ�����ˮ������װ��̼���ƹ�����ձ���ò�����____________��ʹ���ܽ⣬����ȴ�����¡�
�ܴ洢������õ���Һװ���Լ�ƿ��������Ƥ����___________���ŵ�ָ���ĵط���
(3)ȡ50g��Һ����ε����������Ƶ�̼������Һ������pH����̽����������⣬����������[pHΪ�����꣬ʱ��s(��)Ϊ������]��
(1)��״ʯ��ʯ������ϡ���ᷴӦ�������ݳ������Ժ���,�����Һ��pH=2������Һ�� ___________�ԣ���Һ����������Ҫ������________________��
(2)�����ʵ����Ҫ����Ҫ������������Ϊ26.5%��̼������Һ200g
�ټ��㣺��Ҫ̼���ƹ��������Ϊ___________g��ˮ�����Ϊ__________mL��ˮ���ܶȽ��ƿ���1g/cm3��
�ڳ���������������ƽƽ���__________����������ƽ�����̣������������� Ȼ������������__________������̼���ƹ��壬ֱ����ƽƽ�⡣
���ܽ⣺����Ͳ��ȡ�����ˮ������װ��̼���ƹ�����ձ���ò�����____________��ʹ���ܽ⣬����ȴ�����¡�
�ܴ洢������õ���Һװ���Լ�ƿ��������Ƥ����___________���ŵ�ָ���ĵط���
(3)ȡ50g��Һ����ε����������Ƶ�̼������Һ������pH����̽����������⣬����������[pHΪ�����꣬ʱ��s(��)Ϊ������]��
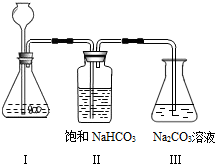
��д��AB�����йػ�ѧ����ʽ____________ ��_______________��_______________��
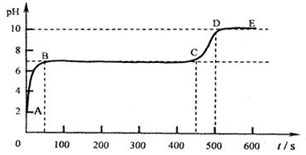
��д��BC��ƽ̨��������____________��
��CD��������ԭ���ǣ�__________________ ��
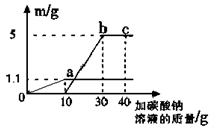
(4)��������ʵ���õ���һ�����ݻ��Ƴ���ͼ��������m��ʵ��õ��ij�����������������������ʾ����̼������Һ��������������ԭ��Һ���Ȼ��Ƶ�����������
��д��BC��ƽ̨��������____________��
��CD��������ԭ���ǣ�__________________ ��
(4)��������ʵ���õ���һ�����ݻ��Ƴ���ͼ��������m��ʵ��õ��ij�����������������������ʾ����̼������Һ��������������ԭ��Һ���Ȼ��Ƶ�����������
��1���� �� H+ ��Ca2+��Cl-
��2����53 ��147�����ձ����룻�۽��裻�����ϱ�ǩ
��3����Na2CO3+2HCl=2NaCl+ H2O+CO2�� ��CaCO3+2HCl=CaCl2+H2O+CO2����
Na2CO3+CaCl2= CaCO3��+2NaCl ���ڳ��ְ�ɫ��������̼������Һ�ʼ��ԣ�������̼������Һʹ��Һ������ǿ
��4��11.1%
��2����53 ��147�����ձ����룻�۽��裻�����ϱ�ǩ
��3����Na2CO3+2HCl=2NaCl+ H2O+CO2�� ��CaCO3+2HCl=CaCl2+H2O+CO2����
Na2CO3+CaCl2= CaCO3��+2NaCl ���ڳ��ְ�ɫ��������̼������Һ�ʼ��ԣ�������̼������Һʹ��Һ������ǿ
��4��11.1%

��ϰ��ϵ�д�
�����Ŀ
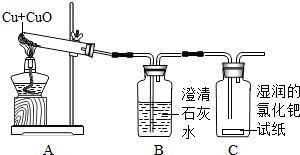 ����ͬѧ��ʵ������ľ̿������ͭ���ֺ�ɫ��ĩ�ڸ����·�Ӧ��ȡ����ͭ����Ӧ�Ļ�ѧ����ʽΪ��C+2CuO
����ͬѧ��ʵ������ľ̿������ͭ���ֺ�ɫ��ĩ�ڸ����·�Ӧ��ȡ����ͭ����Ӧ�Ļ�ѧ����ʽΪ��C+2CuO