��Ŀ����
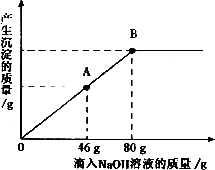
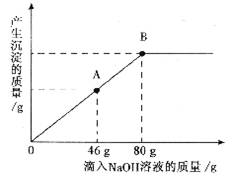
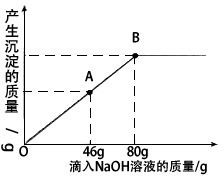
��һ�ձ���ʢ��һ��������MgCO3���壬�����еμ����ʵ��ʾ�����Ϊ10����H2SO4��Һ����ǡ����ȫ��Ӧ���õ�102g��������Һ����������Һ����ε���������������Ϊ10����NaOH��Һ������������������������NaOH��Һ��������ϵ������ͼ��ʾ�����������ش��������⣺
(1)�ڵ���ϡ����ʱ���۲쵽������ʵ��������______________________________��
(2)������NaOH��Һ��ͼ��A��ʱ���ձ�����Һ�ﺬ�е�������(д��ѧʽ) __________��
(3)������10����NaOH��Һ80gʱ(��B��)����ͨ�����㣬���ʱ���ò�������Һ��������(��������ȷ��0.1g)
(2)������NaOH��Һ��ͼ��A��ʱ���ձ�����Һ�ﺬ�е�������(д��ѧʽ) __________��
(3)������10����NaOH��Һ80gʱ(��B��)����ͨ�����㣬���ʱ���ò�������Һ��������(��������ȷ��0.1g)
(1)�������ʲ����ܽ⣻���������(��������ð��)
(2)Na2SO4��MgSO4
(3)�⣺80g10����NaOH��Һ�к�NaOH�������ǣ�80g��10%=8g
�跴Ӧ������Mg(OH)2������Ϊx
MgSO4+2NaOH==Mg(OH)2+Na2SO4
����������80��������58
����������8g��������x

�ձ������ò�������Һ������Ϊ��102g+80g��5.8g=176.2g
��������Һ������Ϊ176.2g��(���������𰸿ɵ÷�)
(2)Na2SO4��MgSO4
(3)�⣺80g10����NaOH��Һ�к�NaOH�������ǣ�80g��10%=8g
�跴Ӧ������Mg(OH)2������Ϊx
MgSO4+2NaOH==Mg(OH)2+Na2SO4
����������80��������58
����������8g��������x

�ձ������ò�������Һ������Ϊ��102g+80g��5.8g=176.2g
��������Һ������Ϊ176.2g��(���������𰸿ɵ÷�)

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ