��Ŀ����
 18���Ķ��������ݣ�����뾻��ˮ�йص����⣺
18���Ķ��������ݣ�����뾻��ˮ�йص����⣺��1����ɴ������ˮ���ϵIJ�Ҷ�����ϵȣ��ò������õĻ�ѧԭ����
����
��2��ClO2����һ������ˮ����������������������ˮ������ClO2����Cl2����������ˮ��������C1O2Ӧ����
��������
��������Ԫ�صĻ��ϼ���+4
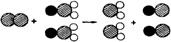
����3���ҹ�����ɹ����Ƴ���ȡClO2���·������䷴Ӧ���۹�����ͼ��ʾ��������
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ���ʾ��ԭ�ӣ���д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ���ʾ��ԭ�ӣ���д����Ӧ�Ļ�ѧ����ʽCl2+2NaClO2�T2NaCl+2ClO2
�����������ݾ�ˮ��֪ʶ���з�����ɴ���ܽ����ϡ���Ҷ�����ʹ��˳�ȥ�����ݻ�ѧʽ�Ķ�������֪��C1O2���ƣ����ݻ��ϼ۹����ڻ������и�Ԫ���������ϼ۵Ĵ�����Ϊ�������û�������ijԪ�صĻ��ϼۣ�����ͼʾ����д����Ӧ�Ļ�ѧ����ʽ��
����⣺��1��ɴ�����˹��˵����ã����Ա����Ϊ�����ˣ�
��2��C1O2�����������ȣ�����Ԫ�صĻ��ϼ�Ϊx������x+��-2����2=0�����x=+4�����Ա����Ϊ���������ȣ�+4��
��3������ͼʾ���Կ�������Ӧ��Ϊһ��Cl2��2��NaClO2�����ɵ���2��NaCl��2��ClO2�����Ա����Ϊ��Cl2+2NaClO2�T2NaCl+2ClO2��
��2��C1O2�����������ȣ�����Ԫ�صĻ��ϼ�Ϊx������x+��-2����2=0�����x=+4�����Ա����Ϊ���������ȣ�+4��
��3������ͼʾ���Կ�������Ӧ��Ϊһ��Cl2��2��NaClO2�����ɵ���2��NaCl��2��ClO2�����Ա����Ϊ��Cl2+2NaClO2�T2NaCl+2ClO2��
���������⿼���˾�ˮ��֪ʶ����ѧʽ�Ķ������������ӻ�ѧ����ʽ��д������ɴ��⣬�����������е�֪ʶ���У���д��Ӧ�Ļ�ѧ����ʽҪ����ͼʾ��ȷд�����ʵĻ�ѧʽ��

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д�
�����Ŀ
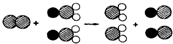
 26���Ķ��������ݣ�����뾻��ˮ�йص����⣺
26���Ķ��������ݣ�����뾻��ˮ�йص����⣺ ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ���д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ���д����Ӧ�Ļ�ѧ����ʽ �Ķ��������ݣ�����뾻��ˮ�йص����⣺
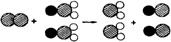
�Ķ��������ݣ�����뾻��ˮ�йص����⣺ ��ʾClԭ�ӣ�
��ʾClԭ�ӣ� ��ʾNaԭ�ӣ�
��ʾNaԭ�ӣ� ��ʾOԭ�ӣ������÷��ű�ʾ��Ӧ����
��ʾOԭ�ӣ������÷��ű�ʾ��Ӧ����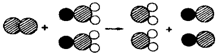 �Ķ��������ݣ�����뾻��ˮ�йص����⣺
�Ķ��������ݣ�����뾻��ˮ�йص����⣺ ��ʾClԭ�ӣ�
��ʾClԭ�ӣ� ��ʾNaԭ�ӣ�
��ʾNaԭ�ӣ� ��ʾOԭ�ӣ������÷��ű�ʾ��Ӧ����______��
��ʾOԭ�ӣ������÷��ű�ʾ��Ӧ����______�� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ���д����Ӧ�Ļ�ѧ����ʽ ��
��ʾ��ԭ�ӣ���д����Ӧ�Ļ�ѧ����ʽ ��