��Ŀ����
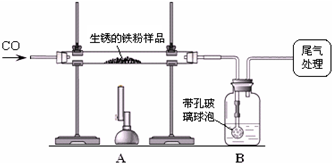
ѧ������ԭ����С�����ô�ԭ���ⶨijһ����������������������������������ȡm1 g�����������Ʒ������ͼ��ʾװ�ý���ʵ�飬�ⶨ����Ʒ�����������������������ⰴ��������������

������ʾ�����ײ������ݿ���ʹҺ��������ֽӴ�
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽ��________��
��2��ijͬѧ��ͨ��Bװ�÷�Ӧǰ��������仯���ⶨ����Ʒ��������������������B�е�����Լ���________������ţ���
�ٳ���ʯ��ˮ���� ����������Ũ��Һ���� ��ϡ���ᡡ�� ��ˮ
ʵ��ʱB�з�Ӧ�Ļ�ѧ����ʽ��________��
��3��ʵ�鿪ʼʱ��Ӧ��ͨһ���CO����ȵ�Ŀ����________��ֹͣ���Ⱥ�Ҫ����ͨһ���CO��Ŀ����________��ʵ������У�CO�������Ϊ��Ӧ���⣬������������________��
��4����ȫ��Ӧ��ͬѧ�Ƶ����۵�����Ϊm2 g��ͬʱ���װ��B����m3 g����������Ʒ������������������Ϊ________��
��5��ʵ���������Ҫ����β��������ԭ��________��
�⣺��1��һ����̼��ԭ�������Ļ�ѧ��Ӧʽ�ǣ�3CO+Fe2O3  2Fe+3CO2���ʴ�Ϊ��3CO+Fe2O3
2Fe+3CO2���ʴ�Ϊ��3CO+Fe2O3  2Fe+3CO2��
2Fe+3CO2��
��2���ܺܺõ����ն�����̼����������������Ũ��Һ��������ͨ��Bװ�÷�Ӧǰ��������仯���ⶨ����Ʒ��������������������Ӧѡ�õ�����Լ��Ǣ���������Ũ��Һ�����������̼�Ļ�ѧ��Ӧʽ�ǣ�CO2+2NaOH=Na2CO3+H2O���ʴ�Ϊ���ڡ�CO2+2NaOH=Na2CO3+H2O��
��3����ΪCO���п�ȼ�ԣ���������������ը������ͨCO��Ŀ���ǣ��ų��������ڵĿ�������ֹ����ʱ������ը��
����ԭ�����������¶Ƚϸߵ���������������е������������±����������Ե�ֹͣ���Ⱥ��м���ͨCO���Թ���ȴ��Ŀ���ǣ���ֹ�����±�������
CO��������Ӧ���⣬���ܶ�ȡ�������е���ʹ������������ԭ��Ӧ����˻�������ԭ����
��4��װ��B����m3g��˵����m3g������̼���ɣ��������ǿ���������m3g������̼��Ҫ����������ΪX
3CO+Fe2O3 2Fe+3CO2
2Fe+3CO2
160 132
X m3g
 =
= X=
X=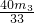
������Ʒ�к�������������������= ��100%=
��100%= %
%
�ʴ�Ϊ��������Ʒ�к�������������������
 %
%
�� 5����ΪCO��һ���ж������壬��Ҫ����β����������Ϊ��CO�ж�������Ⱦ������
�ʴ�Ϊ��
��1��3CO+Fe2O3 2Fe+3CO2 ��2����CO2+2NaOH=Na2CO3+H2O ��3���ų��������ڵĿ�������ֹ����ʱ������ը����ֹ�����±�����������ԭ�� ��4��
2Fe+3CO2 ��2����CO2+2NaOH=Na2CO3+H2O ��3���ų��������ڵĿ�������ֹ����ʱ������ը����ֹ�����±�����������ԭ�� ��4�� %
%
��5��CO�ж�������Ⱦ����
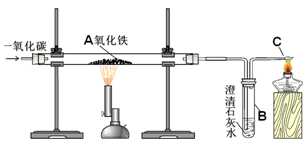
��������1������һ����̼��ԭ������д����ѧ��Ӧʽ��
��2����������Ũ��Һ���Ժܺõ����ն�����̼���壬���ǿ���ͨ��������ѧ��Ӧǰ���Bװ���������ͻ�ѧ��Ӧʽ������Ʒ��������������������
��3�����ݲ������輰ע��������������ӵ���ʧ���ĽǶȷ���CO�����ã�
��4������װ��B����m3g���������������������Ȼ�����������Ʒ������������������������������
��5����CO�Ķ��Է�����
����������ͨ��һ����̼��ԭ�������ķ�Ӧ��������ػ�ѧ��Ӧʽ����д��ʵ��ע������ͼ��㣬Ҫͬѧ�Ǿ����ۺϵĻ�ѧ֪ʶ���ſ����ô��⣮
 2Fe+3CO2���ʴ�Ϊ��3CO+Fe2O3
2Fe+3CO2���ʴ�Ϊ��3CO+Fe2O3  2Fe+3CO2��
2Fe+3CO2����2���ܺܺõ����ն�����̼����������������Ũ��Һ��������ͨ��Bװ�÷�Ӧǰ��������仯���ⶨ����Ʒ��������������������Ӧѡ�õ�����Լ��Ǣ���������Ũ��Һ�����������̼�Ļ�ѧ��Ӧʽ�ǣ�CO2+2NaOH=Na2CO3+H2O���ʴ�Ϊ���ڡ�CO2+2NaOH=Na2CO3+H2O��
��3����ΪCO���п�ȼ�ԣ���������������ը������ͨCO��Ŀ���ǣ��ų��������ڵĿ�������ֹ����ʱ������ը��
����ԭ�����������¶Ƚϸߵ���������������е������������±����������Ե�ֹͣ���Ⱥ��м���ͨCO���Թ���ȴ��Ŀ���ǣ���ֹ�����±�������
CO��������Ӧ���⣬���ܶ�ȡ�������е���ʹ������������ԭ��Ӧ����˻�������ԭ����
��4��װ��B����m3g��˵����m3g������̼���ɣ��������ǿ���������m3g������̼��Ҫ����������ΪX
3CO+Fe2O3
 2Fe+3CO2
2Fe+3CO2160 132
X m3g
 =
= X=
X=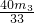
������Ʒ�к�������������������=
 ��100%=
��100%= %
%�ʴ�Ϊ��������Ʒ�к�������������������
 %
%�� 5����ΪCO��һ���ж������壬��Ҫ����β����������Ϊ��CO�ж�������Ⱦ������
�ʴ�Ϊ��
��1��3CO+Fe2O3
 2Fe+3CO2 ��2����CO2+2NaOH=Na2CO3+H2O ��3���ų��������ڵĿ�������ֹ����ʱ������ը����ֹ�����±�����������ԭ�� ��4��
2Fe+3CO2 ��2����CO2+2NaOH=Na2CO3+H2O ��3���ų��������ڵĿ�������ֹ����ʱ������ը����ֹ�����±�����������ԭ�� ��4�� %
%��5��CO�ж�������Ⱦ����
��������1������һ����̼��ԭ������д����ѧ��Ӧʽ��
��2����������Ũ��Һ���Ժܺõ����ն�����̼���壬���ǿ���ͨ��������ѧ��Ӧǰ���Bװ���������ͻ�ѧ��Ӧʽ������Ʒ��������������������
��3�����ݲ������輰ע��������������ӵ���ʧ���ĽǶȷ���CO�����ã�
��4������װ��B����m3g���������������������Ȼ�����������Ʒ������������������������������
��5����CO�Ķ��Է�����
����������ͨ��һ����̼��ԭ�������ķ�Ӧ��������ػ�ѧ��Ӧʽ����д��ʵ��ע������ͼ��㣬Ҫͬѧ�Ǿ����ۺϵĻ�ѧ֪ʶ���ſ����ô��⣮

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ
 ѧ������ԭ����С�����ô�ԭ���ⶨijһ������������������
ѧ������ԭ����С�����ô�ԭ���ⶨijһ������������������![]() ����������������ȡm1 g�����������Ʒ������ͼ��ʾװ�ý���ʵ�飬�ⶨ����Ʒ�����������������������ⰴ��������������
����������������ȡm1 g�����������Ʒ������ͼ��ʾװ�ý���ʵ�飬�ⶨ����Ʒ�����������������������ⰴ��������������
|
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽ��___________________��
��2��ijͬѧ��ͨ��Bװ�÷�Ӧǰ��������仯���ⶨ����Ʒ��������������������
B��![]() ������Լ���_____������ţ���ʵ��ʱB�з�Ӧ�Ļ�ѧ����ʽ��_____��
������Լ���_____������ţ���ʵ��ʱB�з�Ӧ�Ļ�ѧ����ʽ��_____��
�� ����ʯ��ˮ �� ��������Ũ��Һ �� ϡ���� �� ˮ
��3������ʵ������У�CO�������Ϊ��Ӧ���⣬�����������ǣ��� ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը���� ֹͣ���Ⱥ�ֹA�������ﱻ������B�е���Һ������A�У���______________________��
��4����ȫ��Ӧ��ͬѧ�Ƶ����۵�����Ϊm2 g��ͬʱ���װ��B����m3 g����������Ʒ������������������Ϊ_____________________��