��Ŀ����
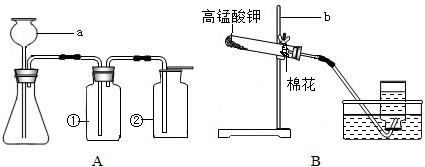
ͼA��B��ʵ���ҳ�������ȡ�����װ�ã�������ѧ֪ʶ�ش��������⣺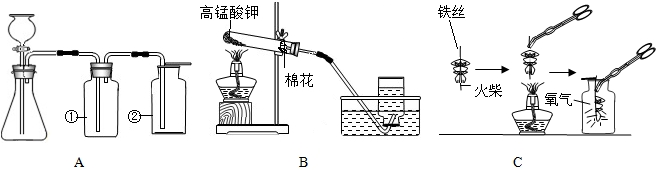
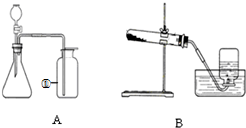
��1��װ��A�Тڵ�����������
��2��װ��B��ʾ�����ø��������ȡ���������л�ȱ�ٵ�������
��3��װ��A�����Եļ��飺�ȹرշ�Һ©��������
��4��ͼC������״��˿��ĩ��ϵһ������������
��5��С������˿��������ȼ��Ϊʲô������������̽�����±���������þ���Ͳ�ͬ��̼������˿��þ������˿ֱ����Ϊ0.4mm������������ȼ��ʱ��ʵ������ļ�¼����������ش�
| ���� | þ�� | ��̼0.05%����˿ | ��̼0.2%����˿ | ��̼0.6%����˿ |
| ȼ��ʱ ������ |
����ȼ�գ����� ҫ�۰⣬���� |
����ȼ�� ���ٻ��� |
����ȼ�� �������� |
��δ� |
��˿��������ȼ��Ϊʲô��������䣿ԭ��
��������1�����ݷ���װ�õ�ѡ��������������ʵ�����Ʒ������жϣ�
��2������ȳ��ƾ��ƣ��ڲ��¶Ƚ��ͣ�ѹǿ��С��ˮ�۵�ˮ�ͻᵹ�������Թܣ�����Թܱ��ѣ�
��3�����������Եķ���һ���Ǹ���ѹǿ�ı仯��ȷ���ģ�
��4����ȼ�յ����� ���п��ǣ�
��5���۲�ͼ���������Ƚϣ�
��2������ȳ��ƾ��ƣ��ڲ��¶Ƚ��ͣ�ѹǿ��С��ˮ�۵�ˮ�ͻᵹ�������Թܣ�����Թܱ��ѣ�
��3�����������Եķ���һ���Ǹ���ѹǿ�ı仯��ȷ���ģ�
��4����ȼ�յ����� ���п��ǣ�
��5���۲�ͼ���������Ƚϣ�
����⣺
��1��Aװ�����ڹ����Һ�岻���������壬����������ʵ�����Ʒ���֪��˫��ˮ�Ͷ������̷����������ʴ�Ϊ������ƿ��MnO2��H2O2����ʵ������̼��ƺ�ϡ�����ƶ�����̼������װ�����ڹ����Һ�岻���������壬�ʴ�Ϊ��A��
��2������ȳ��ƾ��ƣ��ڲ��¶Ƚ��ͣ�ѹǿ��С��ˮ�۵�ˮ�ͻᵹ�������Թܣ�����Թܱ��ѣ�
���ʴ�Ϊ���ƾ��ƣ�2KMnO4
K2MnO4+MnO2+O2�����Ƚ������Ƴ�ˮ�棬��Ϩ��ƾ��ƣ�
��3���ȹرշ�Һ©��������Ȼ���ˮ��ûƿ���е��ܵ�ĩ�ˣ�Ŀ���Ƿ�ֹ����������ȥ��Ȼ������ƿ�Ӣ٣������ڲ��¶����ߣ��������ͣ������©��������Կ�������ð�����ʴ�Ϊ��Ȼ���ˮ��ûƿ���е��ܵ�ĩ�ˣ�ƿ���е��ܿ�û������ð����
��4�����ȼ���ͷ�����������ﵽ��˿���Ż�㣬�ʴ�Ϊ����ȼ��˿���ṩ������ʹ�¶����ߣ��ﵽ��˿���Ż��Ⱥ������ɣ���
��5������ͼ��������֪��ÿ�����������ȼ�գ����ź�̼�������࣬���������࣬�ʴ�Ϊ���������䣻��˿�к���̼��
��1��Aװ�����ڹ����Һ�岻���������壬����������ʵ�����Ʒ���֪��˫��ˮ�Ͷ������̷����������ʴ�Ϊ������ƿ��MnO2��H2O2����ʵ������̼��ƺ�ϡ�����ƶ�����̼������װ�����ڹ����Һ�岻���������壬�ʴ�Ϊ��A��
��2������ȳ��ƾ��ƣ��ڲ��¶Ƚ��ͣ�ѹǿ��С��ˮ�۵�ˮ�ͻᵹ�������Թܣ�����Թܱ��ѣ�
���ʴ�Ϊ���ƾ��ƣ�2KMnO4
| ||
��3���ȹرշ�Һ©��������Ȼ���ˮ��ûƿ���е��ܵ�ĩ�ˣ�Ŀ���Ƿ�ֹ����������ȥ��Ȼ������ƿ�Ӣ٣������ڲ��¶����ߣ��������ͣ������©��������Կ�������ð�����ʴ�Ϊ��Ȼ���ˮ��ûƿ���е��ܵ�ĩ�ˣ�ƿ���е��ܿ�û������ð����
��4�����ȼ���ͷ�����������ﵽ��˿���Ż�㣬�ʴ�Ϊ����ȼ��˿���ṩ������ʹ�¶����ߣ��ﵽ��˿���Ż��Ⱥ������ɣ���
��5������ͼ��������֪��ÿ�����������ȼ�գ����ź�̼�������࣬���������࣬�ʴ�Ϊ���������䣻��˿�к���̼��
����������������ʵ�����Ʒ�����ʵ���������������ѧ�����ͼ���ķ�����

��ϰ��ϵ�д�
�����Ŀ
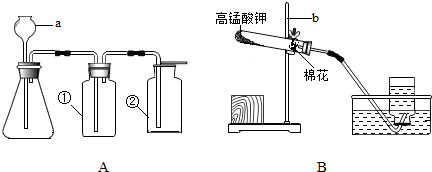
 ��2010?�ɽ���һģ����ͼA��B��ʵ���ҳ��õ���ȡ����װ�ã�������ѧ֪ʶ�ش��������⣺
��2010?�ɽ���һģ����ͼA��B��ʵ���ҳ��õ���ȡ����װ�ã�������ѧ֪ʶ�ش��������⣺